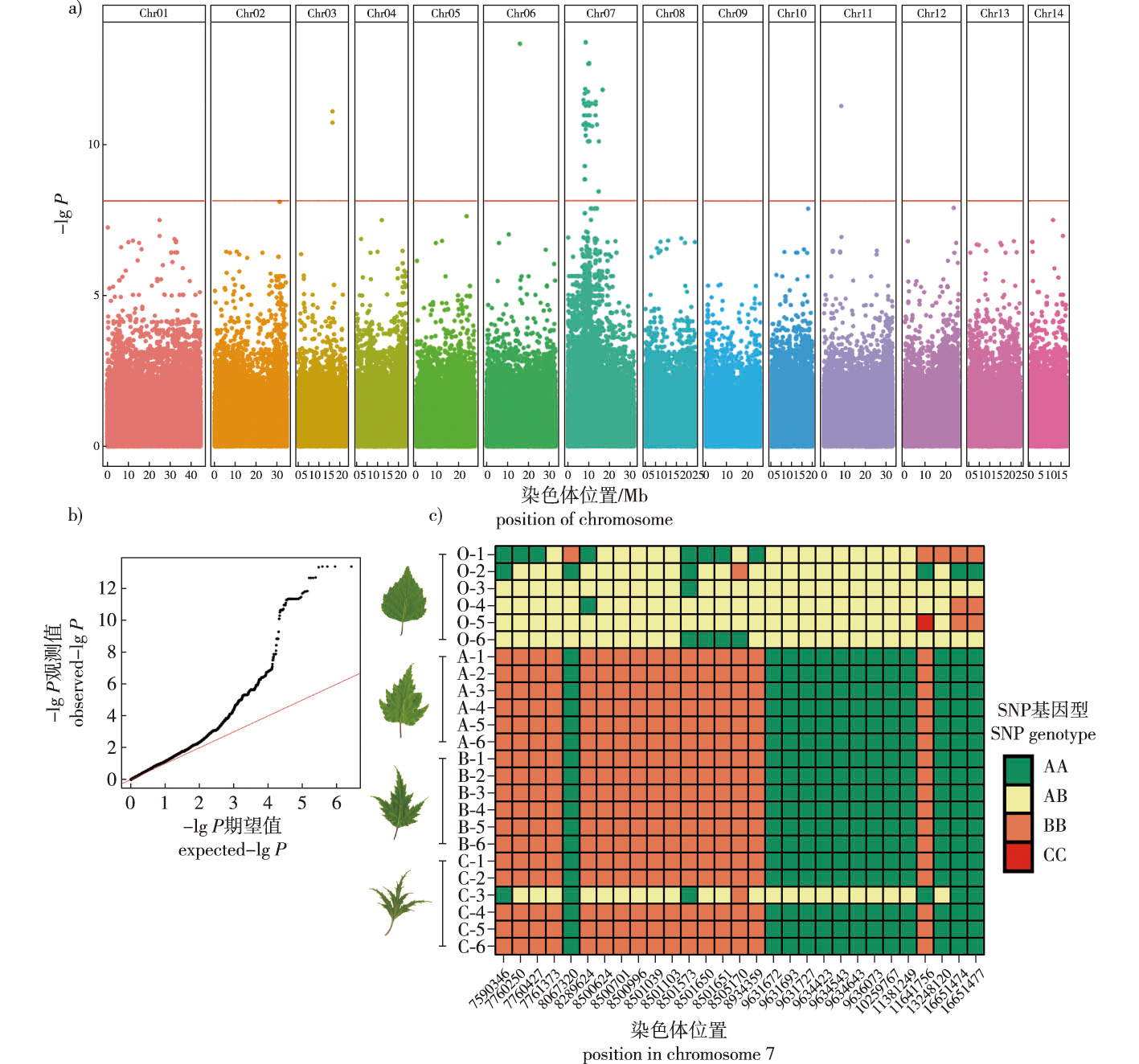
【目的】针对调控裂叶桦( Betula pendula ‘Dalecarlica’)叶形的基因进行初步定位,筛选并注释可能的候选基因,为后续的验证实验和机理研究奠定基础。【方法】以来自1株裂叶桦母树的24株子代个体为试验材料,其包括了正常叶、浅裂叶、深裂叶等类型,扫描每单株的5个叶片,使用衡量叶片不规则度的指标计算每株个体的叶形不规则度,分析个体之间的差异显著性,测定每单株RNA-Seq数据,分析其中的SNP/InDel信息,并结合叶片不规则度进行关联分析,划定关联区。根据RNA-Seq数据分析实验材料全基因组的基因表达量,计算基因差异显著性,继而挑选出关联区中的差异基因。将关联区中受关联性SNP影响的基因和差异表达的基因作为候选基因,使用在线数据库进行基因功能注释。【结果】试验群体叶形不规则度的变异系数达60.51%,满足关联分析的需求。对实验群体全基因组中1 367 359个SNP/InDel标记的关联分析结果显示,裂叶桦叶形调控基因所在的关联区长9.18 MbP,包含400个基因,关联区内61个关联性SNP影响了17个基因的编码。基因表达量分析结果显示,关联区中有25个基因在不同叶形桦树之间存在极显著差异(P<0.01)。对上述候选基因进行功能注释,根据注释结果,预测其中3个转录因子和1个生长素转运蛋白可能是调控裂叶桦叶形的关键基因。【结论】调控裂叶桦叶片分裂性状的基因定位在7号染色体7466344—16651477的区间,包含候选基因40个。根据基因注释结果,建议后续研究重点针对其中3个转录因子和1个生长素外排体基因开展。
【Objective】 As a variety of Betula pendula, B. pendula ‘Dalecarlica’ exhibits lobed shaped split leaves, which have significant application value in horticultural ornamental, breeding, and plant developmental biology research. Understanding the mechanisms of leaf shape variation in B. pendula ‘Dalecarlica’ has academic value in the field of genetics, and identifying the genes corresponding to this trait is important for further research on this topic. This study aimed to preliminarily locate and identify the genes regulating the leaf shape of B. pendula ‘Dalecarlica’, and screen and annotate potential candidate genes, thus laying the groundwork for subsequent validation experiments and mechanistic research. 【Method】 A total of 24 offspring individuals from a single B. pendula ‘Dalecarlica’ maternal tree were selected as experimental materials. This half-sibling family included the offspring of individuals with different leaf types, including normal, week-lobed, lobed and strong-lobed leaves. Five leaves were collected from each plant and scanned. Leaf shape irregularity degree (Dli) was measured as Dli=4πS/C2, and which for each individual was calculated. The significance of Dli differences among individuals was analyzed. RNA-Seq data were obtained for each individual, and SNP/InDel information was called. Using the degree of leaf shape irregularity as a phenotype, an association analysis was performed using a generalized linear model (GLM). The association area were delineated based on SNPs/InDels with significant association signals. Furthermore, gene expression levels of the entire genome in the experimental materials were analyzed based on RNA-Seq data. The significance of gene transcription level differences was calculated, and differentially expressed genes (DEGs) within the association area were identified. SNPs/InDels with association P values smaller than the Bonferroni threshold were considered associated SNPs/InDels. Genes affected by associated SNPs/InDels and differentially expressed genes were considered candidate genes. Gene functional annotations were performed using online databases. 【Result】 The degree of leaf irregularity showed extremely significant differences among different individuals within the experimental population (P < 0.01). The coefficient of variation for leaf shape irregularity in the experimental population was 60.51%, which met the requirements for association analysis. A total of 331 Gb of RNA-Seq data was generated, of which 3 303 530 SNPs/InDels were detected. After filtering, 1 367 359 high-quality polymorphic SNPs/InDels were retained. Using the degree of leaf shape irregularity as the phenotype, association analysis was performed on these SNPs/InDels using a GLM. Association analysis identified 66 SNPs/InDels with significant P values below the Bonferroni threshold. By closely linking these 61 SNPs/InDels, the association area containing the leaf shape-regulating genes of B. pendula ‘Dalecarlica’ was defined, spanning a length of 9.18 MbP and containing 400 genes. Within this area, 61 associated SNPs/InDels were found to affect the coding of 17 known genes. Analysis of gene expression levels revealed that of the 400 genes within the association area, 25 genes showed extremely significant differences (P < 0.01) between B. pendula, B. pendula ‘Dalecarlica’. Functional candidate gene annotation identified three transcription factors, three proteins involved in cell composition, six proteins involved in protein translation processing, nine protein kinases involved in signal transduction, five enzymes involved in biochemical reactions, three proteins involved in substance transport, one protein involved in the post-transcriptional modification of RNA, one DNA endonuclease/exonuclease, and one protein involved in stomatal regulation. Additionally, eight genes could not be found in the existing online databases, and their types and functions remain unknown. Finally, three transcription factors and one auxin carrier protein were considered as possible key genes regulating the leaf shape of B. pendula ‘Dalecarlica’. 【Conclusion】 The gene locus responsible for regulating the splitting trait of B. pendula ‘Dalecarlica’ leaves was mapped to the interval of chromosome 7:7466344-16651477, with a total of 40 candidate genes located within this association area. Based on the gene annotation results, future studies should focus on the three identified transcription factors and one auxin carrier protein.
 PDF(2557 KB)
PDF(2557 KB)


 PDF(2557 KB)
PDF(2557 KB)
 PDF(2557 KB)
PDF(2557 KB)