 PDF(2127 KB)
PDF(2127 KB)


H2S供体NaHS对渗透胁迫下美国剑麻干旱适应性及抗氧化特性的影响
沈瑒, 狄晶晶, 陈颖, 冯凯, 陆锦玲, 胡宇辰
南京林业大学学报(自然科学版) ›› 2024, Vol. 48 ›› Issue (2) : 121-128.
 PDF(2127 KB)
PDF(2127 KB)
 PDF(2127 KB)
PDF(2127 KB)
H2S供体NaHS对渗透胁迫下美国剑麻干旱适应性及抗氧化特性的影响
Effects of H2S donor NaHS on the adaptability and antioxidant properties of Agave americana plantlets under an in vitro culture of osmotic stress
【目的】 美国剑麻(Agave americana)是热带地区一种具有高经济价值的、重要的景天科酸代谢(CAM) 植物,研究其干旱适应性可为CAM植物的耐旱机制研究提供理论依据。【方法】 通过对美国剑麻进行1.0 mmol/L NaHS (NS)、10% PEG(聚乙二醇, PEG10)、20% PEG (PEG20)、1.0 mmol/L NaHS+10%PEG (NS+PEG10)、1.0 mmol/L NaHS+20% PEG (NS+PEG20) 的处理,以不添加PEG和NaHS的处理为对照,研究美国剑麻组培苗对PEG渗透胁迫的响应,以及渗透胁迫下H2S供体NaHS对其渗透调节和抗氧化特性的影响。【结果】 美国剑麻组培苗在20% PEG高浓度处理下仍然存活,具有一定的抗旱能力。但植株出现了鲜质量下降(PEG20处理比对照降低了16.6%)、超微结构发生变化、丙二醛(MDA)和H2O2含量增加等受害症状和氧化胁迫反应。然而美国剑麻可以通过增加脯氨酸、可溶性糖的水平调节渗透压,降低胁迫强度。在高浓度PEG存在下,添加NaHS可降低脯氨酸和H2O2含量,从而减少脯氨酸和H2O2的过度积累;通过调节超氧化物歧化酶(SOD)等5种抗氧化酶活性,提高还原型谷胱甘肽(GSH)含量清除活性氧和活性羰基物,降低美国剑麻的脂质过氧化水平,增强其抗逆性。【结论】 美国剑麻本身具有一定的耐旱性,H2S能够通过调控美国剑麻的渗透调节和抗氧化水平,使其更加适应干旱的环境。
【Objective】 Agave americana is an important crassulacean acid metabolism (CAM) plant with high economic value in tropical areas. Studying its drought adaptability could provide evidence of the drought tolerance mechanism, and support for resource development and engineering research regarding the utilization of CAM plants. 【Method】 In this study, A. americana plantlets were treated with 1.0 mmol/L NaHS (NS), 10% polyethylene glycol (PEG10), 20% PEG (PEG20), 1.0 mmol/L NaHS+10% PEG (NS+PEG10), and 1.0 mmol/L NaHS+20% PEG (NS+PEG20) under an in vitro culture. The responses to PEG osmotic stress were studied and the effects of H2S donor NaHS on osmotic regulation and antioxidant properties in A. americana were investigated. 【Result】 The results showed that the A. americana plantlets could survive under the 20% PEG (high concentration) treatment and had a degree of drought resistance. However, injury symptoms and oxidative stress reactions occurred, with the fresh mass decreasing by 16.6% in PEG20. The cell ultrastructure changed, and the malondialdehyde (MDA) and H2O2 contents increased in PEG20. Despite this, the plantlets regulated osmotic pressure and reduced the stress intensity by increasing the levels of proline and soluble sugars. In the presence of PEG, H2S donor NaHS could reduce the excessive accumulation of proline and H2O2. Furthermore, H2S could activate superoxide dismutase (SOD) and five antioxidases, and increased the glutathione (GSH) content to clear active oxygen species (ROS) and active carbonyl compounds, subsequently enhancing the antioxidant capacity. 【Conclusion】 The A. americana plantlets had a certain degree of drought tolerance. The H2S had an important role in osmotic regulation and regulating antioxidant levels, making the plantlets better adapted to drought conditions.
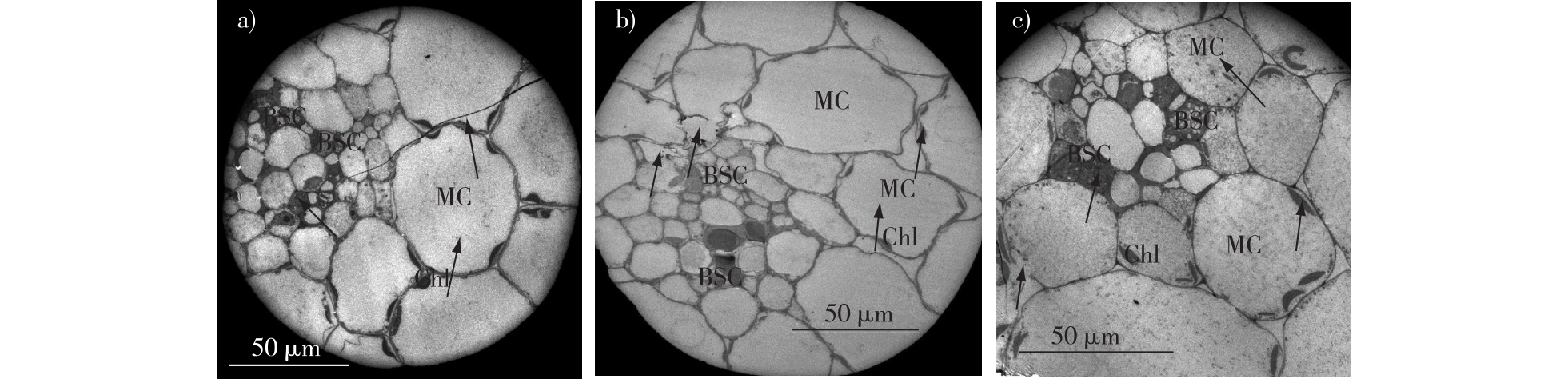
美国剑麻 / 渗透胁迫 / 硫化氢 / 抗旱性 / 渗透调节 / 抗氧化作用
Agave americana / osmotic stress / H2S / drought resistance / osmotic regulation / oxidation resistance
| [1] |
|
| [2] |
陈莉莎. 我国剑麻种质资源纤维强力性能研究[J]. 中国麻业科学, 2023, 45(1):33-40,48.
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
李俊璋, 秦源, 肖强, 等. 景天酸代谢植物分子生物学研究进展及应用潜力[J]. 园艺学报, 2022, 49(12):2597-2610.
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
牟雪姣, 张远兵, 吴燕, 等. 外源H2S缓解黄瓜种子萌发过程中干旱胁迫伤害的生理机制[J]. 西北农业学报, 2018, 27(9):1328-1334.
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
孙晓莉, 张鑫荣, 田寿乐, 等. 外源硫化氢处理对板栗幼苗干旱胁迫抗性的影响[J]. 北方园艺, 2017(15):7-12.
|
| [19] |
李冬, 申洪涛, 王艳芳, 等. 干旱胁迫下外源硫化氢对烤烟幼苗光合荧光参数及抗氧化系统的影响[J]. 西北植物学报, 2019, 39(9):1609-1617.
|
| [20] |
李合生. 植物生理生化实验原理和技术[M]. 北京: 高等教育出版社, 2000.
|
| [21] |
陈建勋, 王晓峰. 植物生理学实验指导[M]. 2版. 广州: 华南理工大学出版社, 2006.
|
| [22] |
|
| [23] |
魏子涵, 袁斌玲, 陈茜, 等. 聚乙二醇处理对‘717’杂交杨组培苗的影响[J]. 森林与环境学报, 2017, 37(4): 412-417.
|
| [24] |
王荔, 张雪, 赵晓珍, 等. 火龙果对干旱胁迫的适应性研究[J]. 热带作物学报, 2020, 41(11):2237-2244.
|
| [25] |
申艳梅. 景天科植物耐旱性及其机理的研究[D]. 呼和浩特: 内蒙古农业大学, 2010.
|
| [26] |
黎远东, 江海霞, 谢丽琼. 植物盐胁迫适应性机制研究进展[J]. 植物遗传资源学报, 2022, 23(6):1585-1593.
|
| [27] |
茶晓飞, 董琼, 段华超, 等. 干旱下白枪杆幼苗生物量及生理活性物质对钙添加的适应性调节[J]. 西北林学院学报, 2023, 38(3):10-17.
|
| [28] |
李林宇, 马靖恒, 张璐瑶, 等. 6-BA预处理对干旱胁迫下越橘生理特性的影响[J]. 分子植物育种, 2023(20):1-11.
|
| [29] |
刘建新, 刘瑞瑞, 刘秀丽, 等. 外源硫化氢对盐碱胁迫下裸燕麦光合碳代谢的调控[J]. 植物生态学报, 2023, 47(3):374-388.
|
| [30] |
张林, 陈翔, 吴宇, 等. 脯氨酸在植物抗逆中的研究进展[J]. 江汉大学学报(自然科学版), 2023, 51(1):42-51.
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
/
| 〈 |
|
〉 |