 PDF(1737 KB)
PDF(1737 KB)


罗汉松叶枯病菌(病原:罗汉松拟盘多毛孢)的生物学特性及其抑制药剂筛选
刘宇茜, 谈家金, 赵梦婷, 丁齐, 戴惠忠, 李春涛
南京林业大学学报(自然科学版) ›› 2025, Vol. 49 ›› Issue (6) : 255-260.
 PDF(1737 KB)
PDF(1737 KB)
 PDF(1737 KB)
PDF(1737 KB)
罗汉松叶枯病菌(病原:罗汉松拟盘多毛孢)的生物学特性及其抑制药剂筛选
Biological characteristics and inhibitor screening of the pathogen: Pestalotiopsis podocarpi causing Podocarpus macrophyllus leaf blight disease
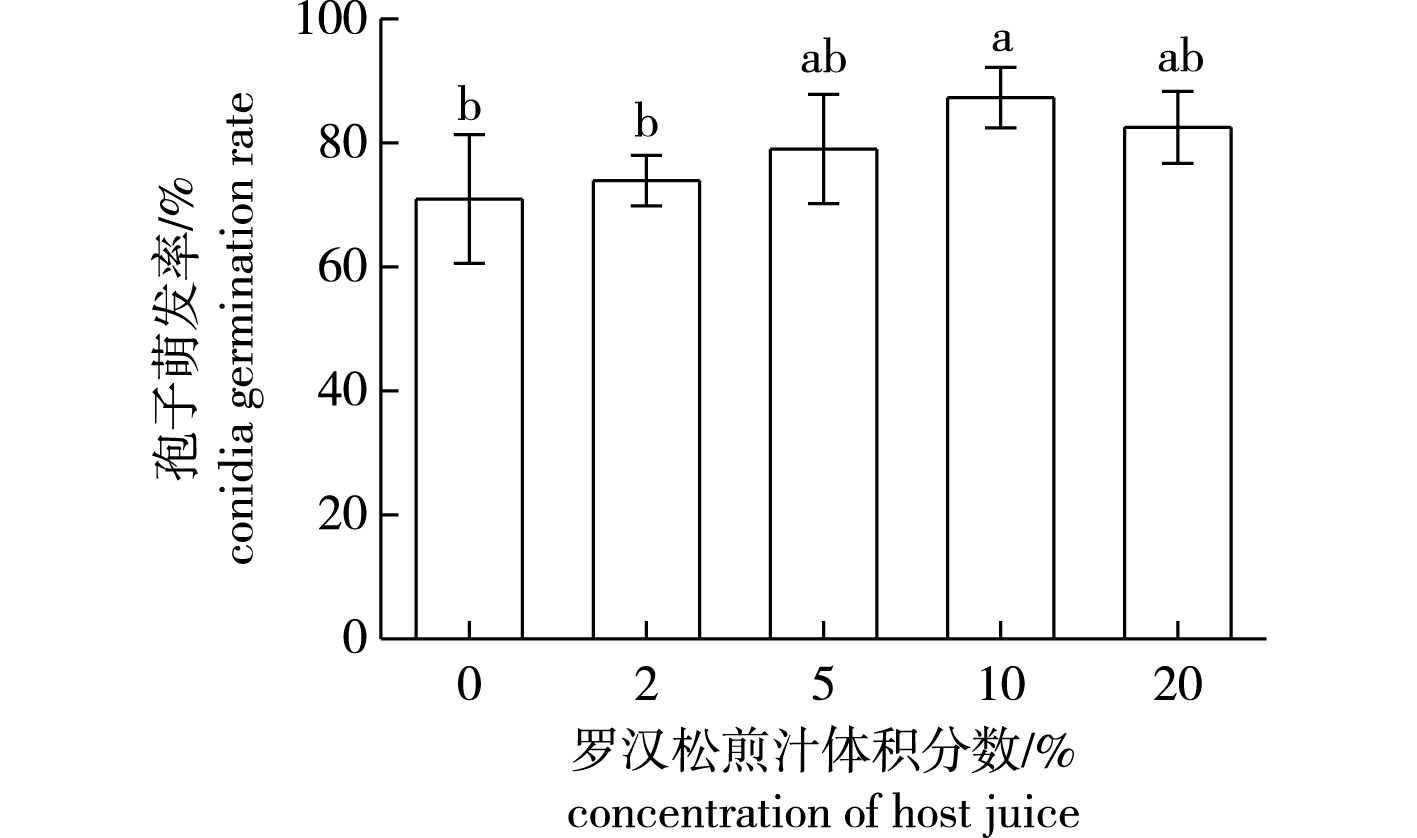
【目的】系统地研究罗汉松(Podocarpus macrophyllus)叶枯病病原物——罗汉松拟盘多毛孢(Pestalotiopsis podocarpi)的生物学特性并进行室内抑制药剂筛选,为该病害有效防控提供参考。【方法】在不同营养条件、温度、pH、光照处理下通过玻片萌发法测定罗汉松拟盘多毛孢孢子的萌发率,以分析其生物学特性。采用含毒介质生长速率法和玻片萌发法对75%(质量分数,下同)百菌清、50%多菌灵、80%代森锰锌、70%甲基托布津等4种药剂进行室内筛选,分别测定了不同药剂在不同浓度下对罗汉松拟盘多毛孢菌丝生长和孢子萌发的抑制作用。通过拟合毒力回归方程计算有效浓度(EC50),筛选得到有效药剂。【结果】寄主植物叶煎汁对病菌孢子萌发具有促进作用,最适浓度(体积分数)为10%;病菌孢子在15~35 ℃均能萌发,最适温度为25 ℃;在pH 6~9时萌发率可达50%以上,以pH为7时萌发率最高;病菌孢子对光照不敏感,光暗交替处理孢子萌发率略高于其他处理。多菌灵对罗汉松拟盘多毛孢菌丝生长抑制效果最好,其有效浓度(EC50)为0.048 9 mg/L;甲基托布津效果次之,EC50为0.850 5 mg/L;而代森锰锌和百菌清的效果较差。代森锰锌对病菌孢子萌发的抑制效果最好,EC50为0.257 3 mg/L。甲基托布津、百菌清和多菌灵的抑制效果依次降低。【结论】适宜病原罗汉松拟盘多毛孢孢子萌发的罗汉松煎汁浓度(体积分数)为10%,最适生长温度和pH分别为25 ℃和7。可选用多菌灵和代森锰锌作为防治罗汉松叶枯病的潜在药剂,但其林间防效还需进一步研究。
【Objective】In order to effectively prevent and control the disease, this study aims to systematically investigate the biological characteristics of the pathogen causing Podocarpus macrophyllus leaf blight disease and screens effective fungicides.【Method】The germination rate of spores was measured under different nutritional conditions, temperatures, pH levels, and light treatments using the glass slide germination method to study the biological characteristics of Pestalotiopsis podocarpi. The four fungicides, including 75% (mass fraction) chlorothalonil, 50% (mass fraction) carbendazol, 80% (mass fraction) mancozeb, and 70% (mass fraction) thiophanate-methyl, were evaluated in vitro for their efficacy in restraining hyphal growth and conidia germination. The toxicity regression equations were fitted to calculate the EC50, and effective fungicides were screened.【Result】The host plant decoction could promote spore germination, with the optimal concentration being 10%. The spores could germinate between 15-35 ℃, with the optimal temperature at 25 ℃. The germination rate could exceed 50% at pH 6-9, peaking at pH 7. The spores were insensitive to light, with alternating light-dark treatment resulting in slightly higher germination rates than other treatments. The most effective one against hyphal growth is carbendazol with the EC50 of 0.048 9 mg/L, followed by thiophanate-methyl with the EC50 of 0.850 5 mg/L. Mancozeb and chlorothalonil are less effective against hyphal growth. Mancozeb demonstrates the strongest inhibitory effect on conidia germination with the EC50 of 0.257 3 mg/L, followed in decreasing order of effectiveness by thiophanate-methyl, chlorothalonil, carbendazol.【Conclusion】The optimal concentration of P. macrophyllus decoction for spore germination of P. podocarpi is 10%. The optimal growth temperature and pH are 25 ℃ and 7, respectively. Carbendazol and mancozeb can be used as potential fungicides for controlling podocarpus (P. macrophyllus) leaf blight disease, while their field control efficacy requires further research.
罗汉松叶枯病 / 罗汉松拟盘多毛孢 / 生物学特性 / 杀菌剂筛选
Podocarpus macrophyllus leaf blight disease / Pestalotiopsis podocarpi / biological characteristics / fungicides screening
| [1] |
黄相玲, 张明月, 朱栗琼, 等. 4种罗汉松叶片形态性状及生理生化特性比较[J]. 江西农业学报, 2018, 30(8):12-15.
|
| [2] |
徐青青. 江西省乡村人居林树种选择与应用研究[D]. 南昌: 江西农业大学, 2022.
|
| [3] |
陈定如. 马尾松、罗汉松、圆柏、侧柏[J]. 广东园林, 2009, 31(6):78-79.
|
| [4] |
薛凯, 李敏. 国家重点保护野生植物介绍——罗汉松属[J]. 生命世界, 2023(4):94-95.
|
| [5] |
陈少萍. 罗汉松栽培与病虫害防治[J]. 中国花卉园艺, 2014(6):44-47.
|
| [6] |
苏勇, 周颖, 张艳明, 等. 罗汉松叶部真菌性病害的病原鉴定[J]. 广东农业科学, 2015, 42(2):64-67,2.
|
| [7] |
蒋伦创. 罗汉松的栽培技术[J]. 广西林业, 2006(6):37-38.
|
| [8] |
孙小茹, 郭芳, 李留振. 观赏植物病害识别与防治[M]. 北京: 中国农业大学出版社, 2017.
|
| [9] |
吴时英, 徐颖. 城市森林病虫害图鉴[M]. 2版. 上海: 上海科学技术出版社, 2019.
|
| [10] |
尹福强, 宋珍, 徐琴, 等. 多花黄精灰霉病病原菌Botrytis deweyae生物学特性及防治药剂筛选[J]. 江苏农业学报, 2024, 40(10):1818-1825.
|
| [11] |
彩万志, 李华平, 徐汉虹. 园林植物病虫害防治[M]. 重庆: 重庆大学出版社, 2015.
|
| [12] |
张新春, 黄荣辉. 1种使孢子快速萌发的好方法[J]. 中国瓜菜, 2008, 21(6):40-41.
|
| [13] |
高国平, 谢皖豫, 王月, 等. 灯台树叶枯病病原菌的鉴定及其生物学特性[J]. 西北林学院学报, 2016, 31(5):194-197.
|
| [14] |
Persimmon canker disease on stems caused by pathogens affects its growth and yield, leads to economic losses, thus it is important to investigate the pathogen in detail to find an effective control method. In this paper, samples from diseased persimmon trees in Yunnan were collected\n to isolate and purify the pathogen, and its biological characteristics were studied. In detail, its morphology was observed and molecular identified. The effects of different carbon and nitrogen sources, temperature, pH and light condition on its growth were analyzed, and the toxicities of\n 6 fungicides to the pathogen were compared. In the results, the pathogens were identified as Pestalotiopsis diospyri, the optimum conditions for its mycelium growth were on persimmon stem decoction medium, temperature 30 °C, light illumination for 24 h and pH at 4. The most suitable\n carbon and nitrogen sources were glucose and sodium nitrate, respectively. The sensitivity test of six fungicides to P. diospyri showed different degrees, the EC50 values of tebuconazole, azoxystrobin, chlorothalonil, difenoconazole, prochloraz, and carbendazim were 0.212,\n 0.313, 4.921, 0.552, 0.035 and 6.175 μg/mL, respectively, revealing that P. diospyri was the most sensitive to prochloraz. In conclusion, P. diospyri grow fastest in acidic and well-lit environments, and prochloraz can be used as a protential fungicide for the treatment\n and prevention of the disease. This paper provides a theoretical basis for further research and field experiment.
|
| [15] |
龙海江, 樊娟. 贵州山茶花灰斑病葡萄牙拟盘多毛孢菌的生物学特性研究初报[J]. 广西植保, 2023, 36(1):14-18.
|
| [16] |
|
| [17] |
王俊凯, 刘峥, 申东晨, 等. 红豆杉健康与感叶枯病针叶内生微生物多样性[J]. 森林工程, 2023, 39(4):10-18.
|
| [18] |
张思雨, 王秋宇, 遇文婧, 等. 风箱果枯枝病原菌生物学特性及16种杀菌剂的毒力比较[J/OL]. 分子植物育种:1-11.
|
| [19] |
侯囡嵩, 陈雅丽, 于得水, 等. 大叶黄杨灰斑病病原菌鉴定、生物学特性及五种杀菌剂对其抑制作用[J]. 植物保护学报, 2019, 46(3):670-678.
|
| [20] |
Leaf blight is a major foliar disease prevalent in all cardamom‐cultivating tracts, manifesting in diverse forms of symptoms. In this study, six symptomatological variants were delineated based on the expression of foliar symptoms in cardamom genotypes (Malabar, Mysore and Vazhukka) and designated as SV 1 to SV 6. Among the symptomatological variants, SV 1, SV 2, SV 3 and SV 6 were more pronounced in Vazhukka, while SV 4 and SV 5 were prominent in Malabar type. Subsequent isolation from the variants yielded whitish colonies, which were correspondingly coded as SV 1 to SV 6. The conidia were fusiform, five‐celled, with three median versicoloured cells, two terminal hyaline cells and measured 23.1–27.25 × 3.84–4.43 μm. The apical cells had two to three tubular, flexuous, unbranched appendages, whereas the basal appendage was single, tubular and unbranched. Based on conidial characteristics and molecular characterization with internal transcribed spacer rDNA region, partial β‐tubulin, translation elongation factor 1 alpha and large subunit (28S) of the nrRNA genes revealed identity of the pathogens as Neopestalotiopsis clavispora. The pathogenicity test was performed on Malabar, Mysore and Vazhukka genotypes, and Koch’s postulates were proved. In‐vitro interaction at three temperature regimes indicated that N. clavispora was inhibitory to Colletotrichum gloeosporioides at 10 and 30°C. Among the fungicides, carbendazim, propiconazole and carbendazim‐mancozeb completely arrested hyphal growth of N. clavispora under in‐vitro conditions. This study constitutes first report on the association of Neopestalotiopsis clavispora with leaf blight disease of small cardamom.
|
| [21] |
詹家绥, 吴娥娇, 刘西莉, 等. 植物病原真菌对几类重要单位点杀菌剂的抗药性分子机制[J]. 中国农业科学, 2014, 47(17):3392-3404.
Site-specific fungicides play an important role in plant disease management. However, frequent applications of the fungicides over a large geographic scale can induce the emergence of resistant strains in the pathogen population. Resistance to fungicides with various modes of action has been documented in many plant fungal pathogens. This review summaries the current advances in understanding of the modes of action in five major classes of site-specific fungicides including methyl benzimidazole carbamate (MBCs), dicarboximide fungicides (DCFs), 14α-demethylase inhibitors (DMIs), quinone outside inhibitors (QoIs) and succinate dehydrogenase inhibitors (SDHIs) and the molecular mechanisms of resistance. Evolutionary process of fungicide resistance and management programme aiming to mitigate the emergence of resistance are also discussed in the review. The target protein of MBCs is β-tubulin, and the resistance in phytopathogenic fungi is linked to point mutation in the target protein. Amino acid substitutions in target protein occur mainly at the positions 50, 167, 198, 200, and 240, and the most frequent mutation is amino acid 198. In general, only one substitution occurs in each resistant isolate. Resistant level varies among isolates with different substitutions. The target protein of DCFs has been unknown, the resistance may be correlated with point mutation in histidine kindnase (OS-related) genes. DMIs inhibit sterol 14α-demethylation step in biosynthesis of ergosterol and resistant mechanisms usually include point mutation of Cyp51 or over-expressions of Cyp51 and transporter genes. But point mutation in Cyp51 is the major mechanism of DMI resistance. Different site mutations or even same site and same amino acid substitutions could lead to different resistance to triazoles. The number of point mutations in Cyp51 varies among fungi, ranging from one mutation to several mutations and different mutations have an additive effect on DMI-resistance. QoIs affect the electron transportation chain by binding to complex III and resistance in this type of fungicides is usually linked to point mutation in Cytb occurring usually at amino acids positions 120-155 and 255-280. The most frequent point mutations are G143A and F129L in Cytb. SDHIs inhibit complex II in electron transportation chain. Its resistance is generally related to point mutation either in SdhB, SdhC or SdhD, but in the majority of pathogens, resistance to SDHIs is due to point mutation in H272 of SdhB. In the contrast, the sites of point mutations in SdhC or SdhD vary among different pathogens.
|
| [22] |
|
| [23] |
毕秋艳, 马志强, 韩秀英, 等. 葡萄霜霉病菌对甲霜灵抗药性治理及其田间抗药菌株遗传稳定性分析[J]. 植物病理学报, 2014, 44(3):302-308.
为了明确葡萄霜霉病菌对甲霜灵的田间抗药性水平发展态势,于轮换用药前后,采用叶盘漂浮法测定了河北、山西、河南3省葡萄主要种植区11个葡萄园试验地葡萄霜霉病菌对甲霜灵敏感性变化动态。结果表明:田间采集的葡萄霜霉病菌对甲霜灵抗药的菌株其抗药性可以稳定遗传;不同地区轮换用药后,葡萄霜霉病菌对甲霜灵的抗药水平变化态势因用药流程的不同而发生相应的变化。采用不同作用机制的杀菌剂轮换或混合用药进行葡萄霜霉病菌对甲霜灵的抗药性治理时,需制定合理的施药流程,并根据抗药性治理的效果不断完善治理措施。
|
| [24] |
王春明, 韩青梅, 黄丽丽, 等. 3种杀菌剂对小麦黑胚病菌的毒力测定及病害防治作用[J]. 西北农林科技大学学报(自然科学版), 2006, 34(7):55-60.
|
| [25] |
Sclerotinia sclerotiorum is a devastating plant pathogen with a broad host range and worldwide distribution. The application of chemical fungicides is a primary strategy for controlling this pathogen. However, under the high selective pressure of chemical fungicides, fungicide resistance has emerged and gradually increased, resulting in the failure to control S. sclerotiorum in the field. Quinofumelin is a novel quinoline fungicide, but its antifungal activities against plant pathogens have been rarely reported. Here, we determined the antifungal activity of quinofumelin against S. sclerotiorum in vitro and in planta. The median effect concentration (EC50) values ranged from 0.0004 to 0.0059 µg ml−1with a mean EC50of 0.0017 ± 0.0009 µg ml−1and were normally distributed (P = 0.402). In addition, no cross resistance was observed between quinofumelin and other fungicides, dimethachlone, boscalid, or carbendazim, which are commonly used to manage S. sclerotiorum. Quinofumelin did not affect glycerol and oxalic acid production of either carbendazim-sensitive or -resistant isolates. Moreover, quinofumelin exhibited excellent protective, curative, and translaminar activity against S. sclerotiorum on oilseed rape leaves. Protective activity was higher than curative activity. Interestingly, quinofumelin inhibited the formation of the infection cushion in S. sclerotiorum, which may contribute to the control efficacy of quinofumelin against S. sclerotiorum in the field. Our findings indicate that quinofumelin has excellent control efficacy against S. sclerotiorum in vitro and in planta as compared with extensively used fungicides and could be used to manage carbendazim- and dimethachlone-resistance in S. sclerotiorum in the field.
|
| [26] |
|
| [27] |
In recent years, the pathogen that causes leaf blast on Amomum tsao-ko repeatedly infected the plants in a large area of Luchun County, Honghe Prefecture, Yunnan Province, China. The disease is caused by the pathogen Pyricularia variabilis. The effects of light, temperature, pH, carbon, and nitrogen sources on the growth of the pathogen were determined, and its sensitivity to six fungicides was determined using the mycelial growth rate method. The optimal conditions for mycelial growth were as follows: temperature: 20–25°C; carbon source: maltose, nitrogen source beef extract, media corn flour, and potato dextrose agar. The mycelia could grow under four types of light conditions: 24 h light, 24 h dark, 12 h light/12 h dark, and 16 h light/8 h dark. In addition, Propiconazole was the most effective inhibitor, with an EC50 value of 0.030 μg/mL, and prochloraz was the second most effective, with an EC50 value of 0.076 μg/mL. It is suggested that the two fungicides should be alternated when used in production. Carbendazim and chlorothalonil were ineffective in inhibiting the fungus, with EC50 values of 6.137 and 3.765 μg/mL, respectively.
|
/
| 〈 |
|
〉 |