 PDF(14148 KB)
PDF(14148 KB)


 PDF(14148 KB)
PDF(14148 KB)
 PDF(14148 KB)
PDF(14148 KB)
红松胚性愈伤组织瞬时转化体系的建立
Establishment of a transient transformation system for embryogenic callus tissue of Pinus koraiensis
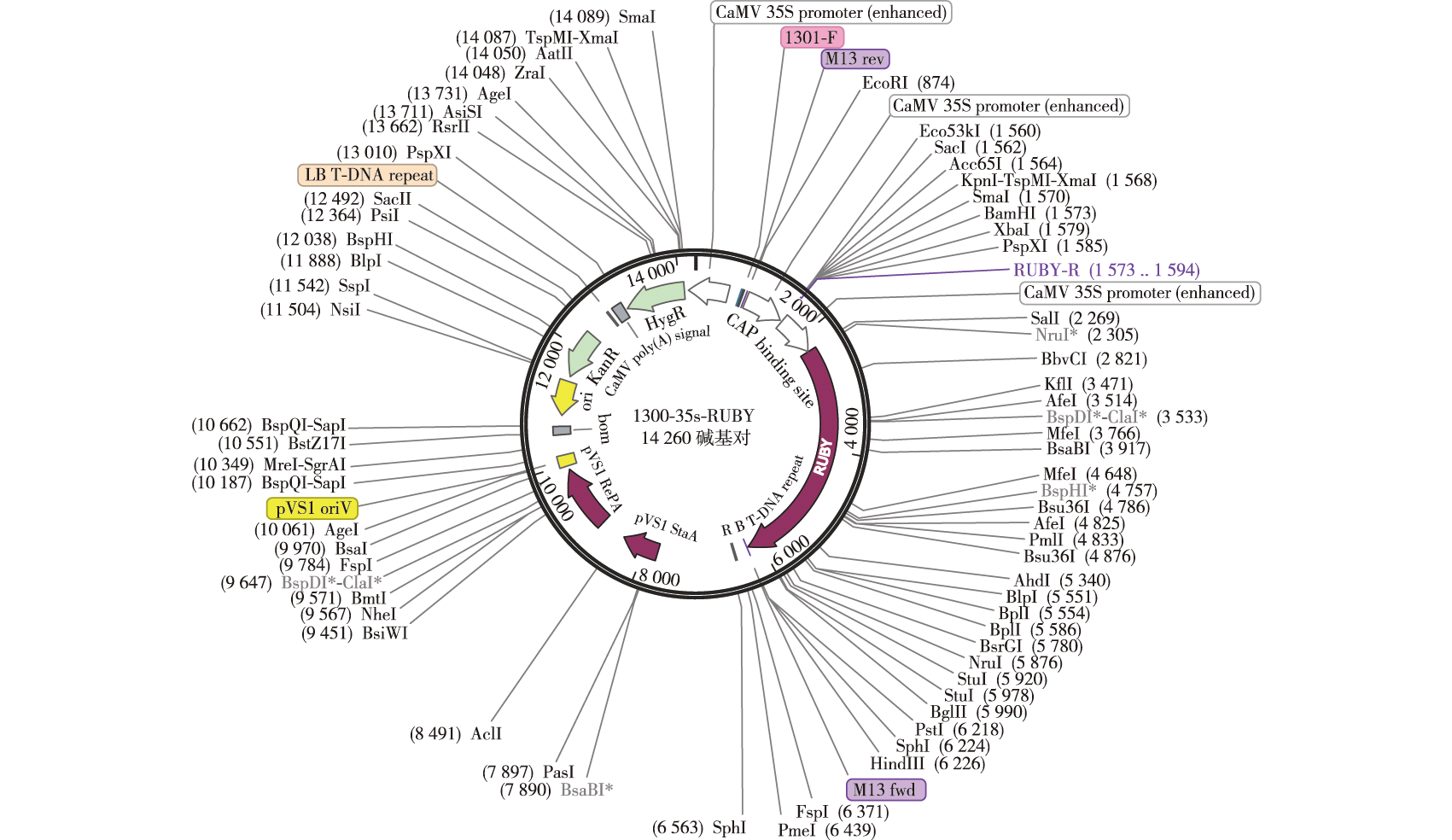
【目的】 优化根癌农杆菌介导的红松(Pinus koraiensis)胚性愈伤组织瞬时转化体系。【方法】以增殖培育10 d的红松胚性愈伤组织为受体材料,利用携带RUBY的pCambia1300载体进行瞬时转化,根据RUBY的表达量筛选最佳侵染液浓度、侵染时间、共培养时间和恢复培养时间。将promoterPkDof5.6-EGFP、promoterPkSHR-RUBY、35S-PkDof5.6-EGFP、35S-PkSHR-EGFP、35S-PkSHR-RUBY载体分别转染红松胚性愈伤组织,验证瞬时转化体系的条件。【结果】瞬时转化后,RUBY表达显著。当侵染菌液浓度(以A600表征)达到0.4、侵染10 min、共培养1 d,无恢复培养期时,RUBY片段的拷贝数最高。基因和启动子验证瞬时转化的定量及半定量结果表示,该条件可以实现红松胚性愈伤组织的高效转化。【结论】本研究成功建立了一种简单、快速、高效、低成本的根癌农杆菌介导的红松胚性愈伤组织瞬时转化体系,并优化了该体系的主要因素,得到最佳的转化条件为:侵染菌液浓度(A600)为0.4,侵染10 min,共培养1 d,无恢复培养期。该体系对红松体胚发生机理的大规模基因功能分析具有重要意义,并可为红松良种选育及规模化扩繁提供参考。
【Objective】 This study aims to optimize a transient transformation system for Pinus koraiensis embryogenic callus mediated by Agrobacterium tumefaciens. 【Method】Using the ten-day cultured embryonic callus of P. koraiensis as the receptor material, transient transformation was carried out with the pCambia1300 vector carrying RUBY. The optimal concentration of infection solution, infection time, co-cultivation time, and recovery culture time were screened based on the expression level of RUBY. The vectors promoterPkDof5.6-EGFP, promoterPkSHR-RUBY, 35S-PkDof5.6-EGFP, 35S-PkSHR-EGFP, and 35S-PkSHR-RUBY were respectively transfected into the embryonic callus of P. koraiensis to verify the conditions of the transient transformation system.【Result】After transient transformation, RUBY expression was significant. When the concentration of the inoculum (A600) reached 0.4, with an infection time of ten minutes and co-cultivation for one day without recovery culture period, the copy number of the RUBY fragment was at its highest. Genes and promoters specific validation of the quantitative and semi-quantitative results of transient transformation indicates that this condition can achieve efficient transformation of mature callus from P. koraiensis. 【Conclusion】This study successfully established a simple, rapid, efficient and low-cost Agrobacterium-mediated transient transformation system for embryogenic callus of P. koraiensis, and optimized the main factors of this system. The best transformation conditions were found to be: an infection bacterial suspension concentration (A600) of 0.4, infection for ten minutes, co-cultivation for one day, with no recovery culture period. This system is of great significance for large-scale gene functional analysis of the somatic embryogenesis mechanism in P. koraiensis and accelerates the breeding and large-scale propagation of high-quality P. koraiensis varieties.
Pinus koraiensis / transient transformation / somatic embryogenesis / molecular breeding
| [1] |
刘德栋. 我国红松良种选育研究进展[J]. 防护林科技, 2017, 30(3):96-99.
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
古文鹏, 贾森泉, 周永明, 等. 艰难梭菌tcdA和tcdB基因绝对定量PCR方法的建立及应用[J]. 中国抗生素杂志, 2025, 50(2):137-143.
|
| [9] |
李玉珠, 余江弟, 丁菲菲, 等. 植物遗传转化中体细胞再生的分子机制及应用研究进展[J]. 草业学报, 2024, 33(2):198-211.
|
| [10] |
李玲, 顾恒, 岳远征, 等. 木本植物瞬时转化体系的研究进展[J]. 分子植物育种, 2020, 18(23):7784-7794.
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
俞瑶, 程华, 陈素梅, 等. 基于农杆菌真空渗透法的菊花瞬时表达系统的优化[J]. 农业生物技术学报, 2023, 31(12):2654-2664.
|
| [18] |
杨海清, 吴凡颖, 王睿琦, 等. 桃愈伤组织和果实瞬时转化体系的优化[J]. 北京农学院学报, 2024, 39(1):13-17,57.
|
| [19] |
闵思源, 陈柏楠, 陈康, 等. 桂花与石楠叶片中瞬时表达体系的建立试验初报[J]. 南方农业, 2020, 14(17):127-128,135.
|
| [20] |
田淑婷, 曹晓云, 时春莹, 等. 农杆菌介导的葡萄风信子愈伤组织遗传转化体系的优化[J]. 草地学报, 2023, 31(4):1234-1241.
|
| [21] |
李晓军, 安轶, 黄李超, 等. 银腺杨84K茎段瞬时转化体系的建立[J]. 林业科学, 2021, 57(4):82-89.
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
安文杰, 张永侠, 原海燕. 红籽鸢尾瞬时表达体系的建立[J]. 分子植物育种, 2020, 18(19):6359-6363.
|
| [26] |
|
| [27] |
常婧, 高行英, 李小东, 等. 农杆菌介导韭菜遗传转化相关因素的研究[J]. 生物技术通报, 2014, 30(1):125-131.
|
| [28] |
唐靖雯, 王宁, 伍程程, 等. 白花玉石籽石榴遗传转化体系的建立[J]. 果树学报, 2024, 41(12):2621-2633.
|
| [29] |
余晓敏, 王亚琴, 刘雨菡, 等. 根癌农杆菌介导万寿菊遗传转化体系的建立[J]. 植物学报, 2023, 58(5):760-769.
|
| [30] |
王冬月, 王如月, 孙茂桐, 等. ‘窄冠白杨1号’遗传转化体系建立与抗虫基因转化[J]. 植物研究, 2024, 44(3):361-369.
|
| [31] |
王梦真, 何锐杰, 邓雅婷, 等. 百香果高频再生及遗传转化体系的建立[J/OL]. 分子植物育种, 2024:1-9.( 2024-04-03). https://kns.cnki.net/KCMS/detail/detail.aspx?filename=FZZW20240329006&dbname=CJFD&dbcode=CJFQ.
|
| [32] |
何旭, 高源, 张群野, 等. 白城小黑杨遗传转化体系建立及其应用[J]. 植物研究, 2023, 43(5):667-678.
|
| [33] |
刘炎, 刘克俭, 王江, 等. 农杆菌介导的针叶树种遗传转化研究进展[J]. 黑龙江农业科学, 2021(4):136-141.
|
| [34] |
|
| [35] |
李菲, 曹永琼, 王纲, 等. 根癌农杆菌介导彩色马蹄莲遗传转化体系的建立及优化[J]. 南方农业学报, 2024, 55(6):1692-1699.
|
/
| 〈 |
|
〉 |