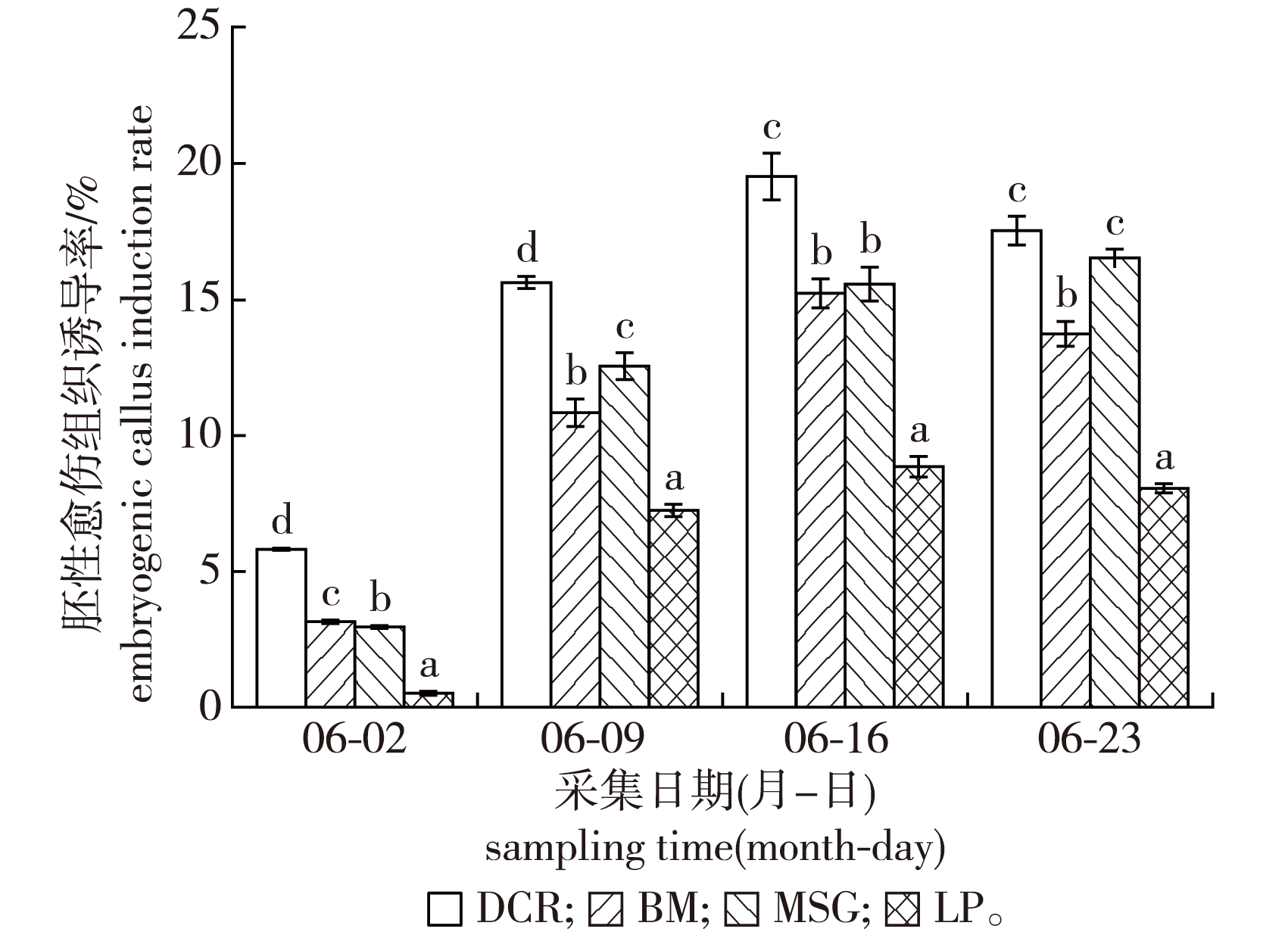
【目的】 对湿地松×加勒比松(Pinus elliottii × P. caribaea)杂交种体细胞胚胎发生的影响因子进行研究,探究不同发育阶段各因子在体细胞胚发生过程中的作用,为建立高效的体细胞胚发生体系,并规模化生产应用提供技术支撑。【方法】以4个杂交组合的湿地松×加勒比松杂交种未成熟合子胚为材料,分析影响胚性愈伤组织诱导的主要因素:基因型、合子胚发育阶段、基本培养基、激素种类和浓度等,筛选生长状态良好的胚性愈伤组织,进行体细胞胚成熟、萌发及植株再生研究,最终获得再生植株。【结果】基因型、合子胚发育阶段、基本培养基和激素组合及浓度等因子均对胚性愈伤组织诱导有显著影响。6月9—23日即裂生多胚至子叶前期阶段为外植体的最佳采集时期,最佳的胚性愈伤组织诱导培养基为DCR+2.0 mg/L 2,4-D+1.0 mg/L BA+0.5 mg/L KT,平均诱导率为19.51%;最佳胚性愈伤组织继代与增殖培养基为改良P6+0.8 m/L 2,4-D+0.5 mg/L BA+0.5 mg/L KT;体细胞胚成熟最佳培养基为改良P6+10.0 mg/L ABA+10.0 mg/L GA+0.2 mg/L 2,4-D+4 000 mg/L 肌醇+2.0 g/L活性炭,成熟培养周期为6~7 周,每mL 密实细胞体积(packed cell volume,PCV)胚性愈伤组织可诱导产生298个成熟体细胞胚;干化处理抑制了体细胞胚的萌发,最佳萌发培养基为1/2 DCR+0.2 mg/L NAA+0.5 mg/L BA+2.0 g/L 活性炭;再将正常萌发的体细胞胚在1/2 DCR +0.1 mg/L NAA+0.5 mg/L BA+2.5 g/L 活性炭培养基上转接培养至长出真叶,苗高3~4 cm时,即可移栽至河沙、珍珠岩、泥炭土体积比为1∶1∶1的基质中,1月后可生长成7~8 cm健壮植株。【结论】通过改进和优化,提出一种更高效的固-液-固交替培养方法,建立完整、高效的湿地松×加勒比松杂交种体细胞胚发生体系,为杂交种的大规模繁殖提供了基础平台,在造林、遗传保护和基于生物反应器的繁殖系统中具有较高应用价值。
【Objective】 The large-scale production and application of the new Pinus elliottii × P. caribaea hybrid requires the application of an efficient somatic embryogenesis system. Immature zygotic embryos were used as explants to investigate which key factors influence somatic embryogenesis during embryogenic callus induction, proliferation, maturation, germination and plantlet acclimatization,establishing a comprehensive protocol for efficient SE propagation of this economically important hybrid.【Method】A total of four representative genotyes of Pinus elliottii × P. caribea were used as starting material to investigate the main factors that affect the induction of embryogenic callus: including genotype, zygotic embryo developmental stage (June 2nd to 23 rd), basic culture medium(DCR, LP, MSG and BM), hormone type and concentration, etc. Well developing embryogenic callus was analyzed for somatic embryo maturation, germination and plant regeneration. Ultimately, regenerated plants were obtained.【Result】All investigated factors (genotype, developmental stage, basic culture medium, hormone (PGR) combination and concentration) were found to have significant effects on embryogenic callus induction. The optimal time to collect explants was from June 9th to 23rd: the multi-embryo division to early cotyledon stage. The optimal embryogenic callus induction medium is DCR with 2.0 mg/L 2,4-D,1.0 mg/L BA and 0.5 mg/L KT. The average induction rate was 19.51%. To maintain the embryonic callus in a proliferative state, capable of differentiation, for a prolonged amount of time, a solid-liquid alternating culture protocol and reduced hormone concentration in the proliferation medium was required. The optimal proliferation medium was modified P6, supplemented with 0.8 mg/L 2,4-D, 0.5 mg/L BA and 0.5 mg/L KT on the solid culture medium and in the first-time liquid culture medium. During the subsequent liquid culture process, the hormone concentration should be gradually reduced. The optimal culture media for somatic embryo maturation was modified P6, with 10.0 mg/L ABA, 10.0 mg/L GA, 0.2 mg/L 2,4-D, 4 000 mg/L inositol and 2.0 mg/L activated carbon(AC). 298 mature somatic embryos were induced per mL of packed cell volume (PCV) embryogenic callus at the maturation culture cycle (6-7 weeks). Drying treatment inhibited the germination of somatic embryos, and the germination rate was best when cultured on 1/2 DCR, supplemented with 0.2 mg/L NAA, 0.5 mg/L BA and 2.0 mg/L activated carbon. Successfully germinating somatic embryos were transferred to the following medium for further cultivation until they reached 3-4 cm in height with true leaves: 1/2 DCR + 0.1 mg/L NAA+ 0.5 mg/L BA +2.5 g/L AC. Finally, the matured plantlets were transplanted and hardened onto a volume ratio of 1∶1∶1 of river sand∶perlite∶peat soil substrate and grown into robust 7-8 cm tall plants over the course of one month. 【Conclusion】In this study a complete and efficient somatic embryogenesis system for the P. elliottii × P. caribaea hybrid has been established. Furthermore, a more efficient method for embryogenic callus maintenance, using solid-liquid-solid alternating culture, has been developed. This system provides a foundational platform for large-scale clonal propagation of hybrid, with potential applications in forestation, genetic conservation, and bioreactor-based propagation systems.
 PDF(8448 KB)
PDF(8448 KB)


 PDF(8448 KB)
PDF(8448 KB)
 PDF(8448 KB)
PDF(8448 KB)