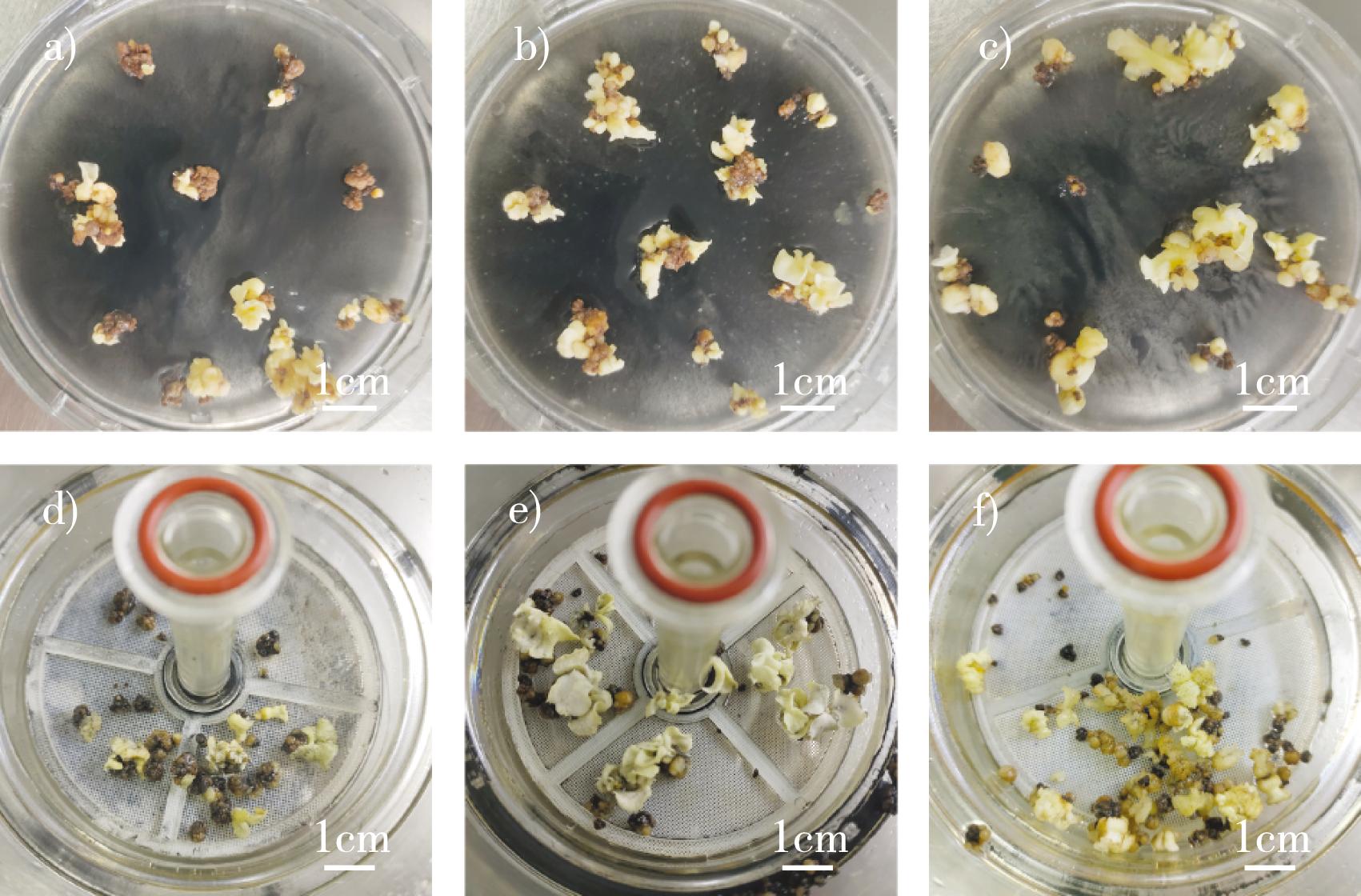
【目的】 建立基于间歇浸没式生物反应器(RITA®)的橡胶树高效体胚发生和增殖体系,为橡胶树体胚苗规模化繁殖奠定基础。【方法】以橡胶树‘热研7-33-97’(Hevea brasiliensis cv. Reyan 7-33-97)花药愈伤组织及次生体胚发生过程中球形胚、心形胚和鱼雷形胚为试验材料,比较RITA®与固体培养的体胚诱导率、正常胚形成率、体胚萌发率及再生率差异,并检测和分析2种培养方式在体胚发育过程中可溶性糖、蛋白质和淀粉含量的变化。【结果】RITA®结合M5培养基,体胚诱导率和诱导系数均显著提高,分别为90%和2.55,正常胚形成率则以RITA®-M4组合最优(40.95%),其余组合无显著差异。在体胚进一步发育过程中,心形胚和鱼雷形胚通过RITA®培养获得的体胚总数和正常胚数均显著高于固体培养,其中鱼雷形胚获得的结果优于心形胚,分别约为153和128,球形胚则更适应固体培养环境;在体胚萌发阶段,RITA®培养显著提高了体胚萌发率,且明显缩短了萌发时间。分析可溶性糖、蛋白质和淀粉含量的变化可知,RITA®培养在多个关键发育阶段具有更高的代谢活性和能量储备效率,为加速体胚成熟和促进体胚萌发提供生理基础。【结论】RITA®培养显著促进橡胶树体胚中后期的发育和成熟,提升体胚质量,缩短再生周期,宜采用“早期固体-中后期RITA®”分阶段培养模式进行橡胶树次生体胚增殖和体胚苗规模化生产。
【Objective】Hevea brasiliensis (rubber tree) plantlets derived from somatic embryogenesis demonstrate enhanced growth vigor and yield potential compared to conventionally grafted seedlings, representing a promising material for the renewal and modernization of rubber plantations. This study aims to establish an efficient system for somatic embryogenesis and proliferation in the rubber tree cultivar ‘Reyan 7-33-97’ using a temporary immersion bioreactor (RITA®), with the goal of improving the production efficiency of somatic embryos and providing a technical foundation for large-scale propagation of high-quality plantlets.【Method】Anther-derived callus and secondary somatic embryos at the globular, heart and torpedo stages were used as experimental materials. Three distinct medium formulations were evaluated for somatic embryo induction from anther-derived calli under both solid and RITA®-based culture conditions. Parameters including somatic embryo induction rate, normal embryo formation rate, germination rate, and plant regeneration rate were systematically compared between the two culture systems. In addition, dynamic changes in key physiological indicators—soluble sugar, protein, and starch—were detected and analyzed throughout the somatic embryogenesis process.【Result】The effectiveness of the RITA® system was highly dependent on the medium composition and the developmental stage of the embryos. During the induction of somatic embryos from anther-derived callus, most combinations of culture methods and media showed no significant differences in the embryo induction coefficient or normal embryo formation rate. However, the RITA®-M5 combination significantly increased the embryo induction rate to 90% and the induction coefficient to 2.55. The highest normal embryo formation rate (40.95%) was achieved using the RITA®-M4 medium. In subsequent developmental stages, hear-stage and torpedo-stage embryos cultured in the RITA® system produced significantly higher numbers of both total and normal somatic embryos compared to solid culture. Torpedo-stage embryos exhibited superior performance relative to heart-stage embryos, yielding totals of 153 and 128 somatic embryos and normal embryos, respectively. In contrast, globular-stage embryos were better adapted to solid culture conditions. Although the total number of somatic embryos induced under RITA® was not significantly different from that under solid culture, the normal embryo formation rate was significantly higher under solid culture (49.03%) than under RITA® (32.30%). During germination, the RITA® system significantly shortened the time required for germination. By day ten, the germination rate under RITA® was nearly 20% higher than that under solid culture, and the shoots emerging from RITA®-cultured embryos were significantly longer. After one month, although no statistically significant difference in plant regeneration rate was observed between the two systems, the RITA® group still exhibited a numerical advantage. Physiological analyses revealed generally similar trends in soluble sugar, protein, and starch content under both culture systems. However, RITA® promoted more rapid consumption of soluble sugars at the heart-shaped stage and supported more efficient accumulation of proteins and starch, providing a physiological basis for accelerated embryo maturation and improved germination efficiency. 【Conclusion】A relatively efficient system for somatic embryo induction and proliferation in rubber tree has been established using the RITA® temporary immersion culture system. This study elucidates the physiological mechanisms by which RITA® enhances somatic embryo maturation and germination, thereby providing both theoretical and technical support for the large-scale propagation of somatic embryo-derived plantlets in H. brasiliensis. Given that RITA® primarily promotes development and germination from the heart stage onward, while the globular stage remains more suitable for solid culture, a phased culture strategy—“solid culture for early stages, followed by RITA® in the mid-to-late stages”—is recommended for the secondary somatic embryo proliferation and large-scale production of somatic plantlets in rubber tree.
 PDF(5796 KB)
PDF(5796 KB)


 PDF(5796 KB)
PDF(5796 KB)
 PDF(5796 KB)
PDF(5796 KB)