 PDF(2724 KB)
PDF(2724 KB)


Effects of the compound inoculation of two arbuscular mycorrhizal(AM) fungi on the resistance of Populus pseudo-cathayana × P. deltoides leaves to Hyphantria cunea
FANG Jing, ZHANG Shuman, YAN Shanchun, WU Shuai, ZHAO Jiaqi, MENG Zhaojun
Journal of Nanjing Forestry University (Natural Sciences Edition) ›› 2024, Vol. 48 ›› Issue (2) : 144-154.
 PDF(2724 KB)
PDF(2724 KB)
 PDF(2724 KB)
PDF(2724 KB)
Effects of the compound inoculation of two arbuscular mycorrhizal(AM) fungi on the resistance of Populus pseudo-cathayana × P. deltoides leaves to Hyphantria cunea
【Objective】 In order to study a new way of biological control of pests on Populus pseudo-cathayana × P. deltoides leaves, the effects of compound inoculation with arbuscular mycorrhizal (AM) fungi on the resistance of P. pseudo-cathayana×P. deltoides leaves to Hyphantria cunea were investigated. 【Method】 One-year-old P. pseudo-cathayana × P. deltoides cuttings were inoculated with Rhizophagus intraradices (RI) and Funneliformis mosseae (FM) by single and mixed inoculation (M) in a greenhouse, with no inoculation (CK) as the control. The chemical defense ability of secondary metabolites, defense enzymes and protease inhibitors in poplar leaves was determined, and the larva of H. cunea was used as a bioassay object to determine its anti-insect effect.【Result】 In 120 days, the mycorrhizal infection rate, arbuscular growth rate and number of vesicles in the root of the M group were higher than those of the FM and RI groups. Meanwhile, the M group could improve the chemical defense ability of the leaves of P. pseudo-cathayana × P. deltoides to a certain extent. The contents of total alkaloids and cellulose and the activities of peroxidase (POD), catalase (CAT), lipoxygenase (LOX), polyphenol oxidase (PPO), chymotrypsin inhibitor (CI) and trypsin inhibitor (TI) in leaves were significantly higher than those in the RI, FM and CK groups (P<0.05). The food intake, fecal output, cellulase activity, acetylcholinesterase (AchE) activity and multifunctional oxidase (MFO) activity of the third instar larvae in the M group were significantly lower than those in the FM, RI and CK groups (P<0.05). The body length, food availability, trypsin activity, carboxylesterase (CarE) activity, and glutathione S-transferase (GSTs) activity of the third instar larvae were significantly higher than those of the RI and FM groups (P<0.05). The food consumption rate and α-amylase activity of the third instar larvae did not significantly differ from those of the RI group, and the body weight of the third instar larvae did not significantly differ from those of the FM group. Body weight, food intake, food consumption rate, cellulase activity, GSTs activity, CarE activity, AchE activity and MFO activity of the fourth instar larvae were significantly lower than those of the FM, RI and CK groups (P<0.05), the fecal output of the fourth instar larvae was significantly lower than that of the RI and CK groups (P<0.05), and the trypsin activity of the fourth instar larvae was significantly higher than that of the other three groups (P<0.05). The body length and food availability of the fourth instar larvae did not significantly differ from those of the RI group, and the α-amylase activity of the fourth instar larvae did not significantly differ from those of the FM and RI groups. The body length, body weight, food intake, fecal output, food utilization, α-amylase activity, cellulase activity, GSTs activity, CarE activity, and MFO activity of the fifth instar larvae were significantly lower than those in the FM, RI and CK groups (P<0.05). The food consumption rate of the fifth instar larvae was significantly lower than that in the FM and RI groups, and the AchE activity of the fifth instar larvae was significantly lower than that in the FM group (P<0.05). The trypsin activity of the fifth instar larvae was significantly higher than that of the other three groups (P <0.05).【Conclusion】 The mixed inoculation of RI and FM could induce the chemical defense performance of P. pseudo-cathayana × P. deltoides leaves in the aspects of secondary metabolites, defense enzymes, and protease inhibitors. The anti-insect performance of combined RI and FM inoculation was better than that of single RI and FM inoculation and no AM fungi inoculation and had certain inhibitory effect on the larva of H. cunea. In practical application, combined RI and FM inoculation can be prioritized.
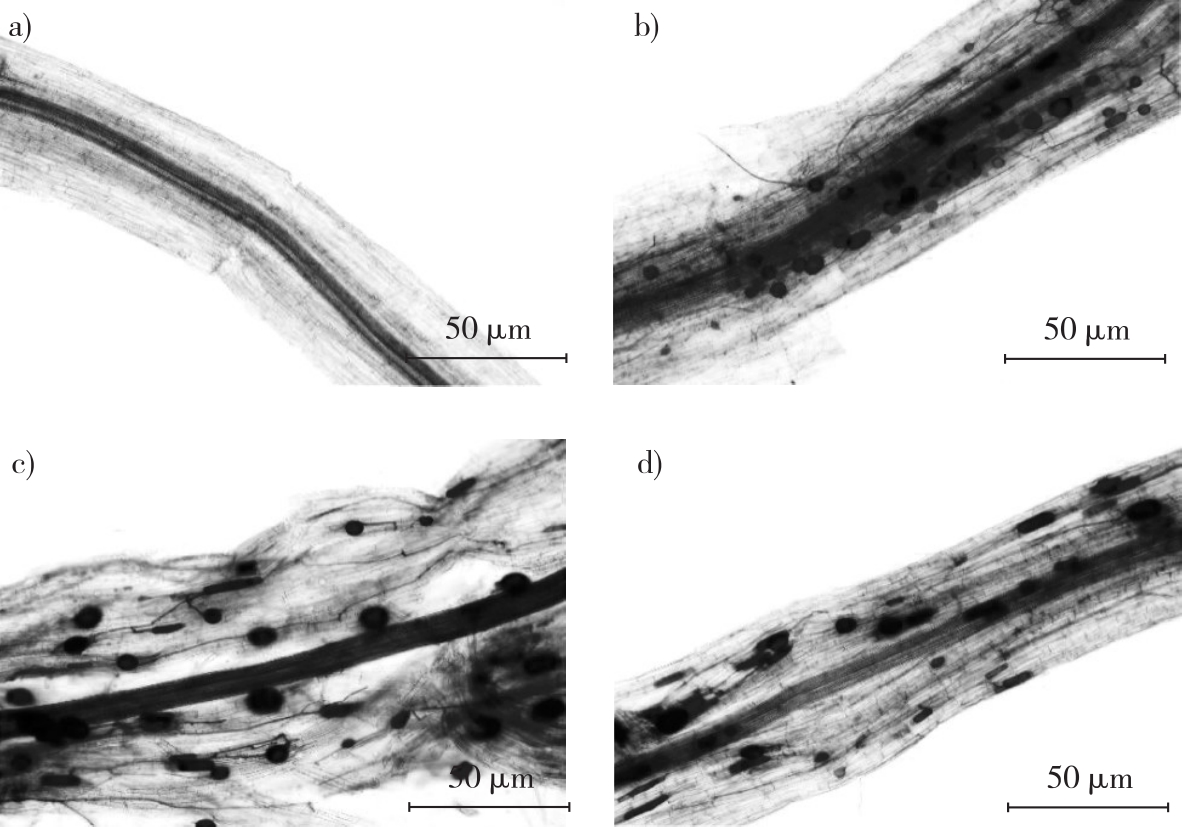
arbuscular mycorrhizal (AM) fungi / Rhizophagus intraradices / Funneliformis mosseae / compound inoculation / Populus pseudo-cathayana × P. deltoids / Hyphantria cunea
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
王幼珊, 刘润进. 球囊菌门丛枝菌根真菌最新分类系统菌种名录[J]. 菌物学报, 2017, 36(7): 820-850.
|
| [5] |
|
| [6] |
张可可, 蒋德明, 余海滨, 等. 接种菌根菌剂对科尔沁沙地4种造林幼苗生长特性的影响[J]. 生态学杂志, 2017, 36(7):1791-1800.
|
| [7] |
|
| [8] |
黄小辉, 陈道静, 冯大兰. 不同基质条件下丛枝菌根真菌对桑树生长的影响[J]. 南京林业大学学报(自然科学版), 2019, 43(3): 9-16.
|
| [9] |
张伟珍, 段廷玉. AM真菌对箭筈豌豆响应豌豆蚜取食的影响[J]. 草地学报, 2019, 27(6): 1518-1525.
|
| [10] |
|
| [11] |
王小菲, 高文强, 刘建锋, 等. 植物防御策略及其环境驱动机制[J]. 生态学杂志, 2015, 34(12): 3542-3552.
|
| [12] |
禹海鑫, 叶文丰, 孙民琴, 等. 植物与植食性昆虫防御与反防御的三个层次[J]. 生态学杂志, 2015, 34(1): 256-262.
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
李虹谕, 卫星, 徐庆祥. 接种丛枝菌根真菌对水曲柳实生苗光合特性和叶片解剖结构的影响[J]. 东北林业大学学报, 2019, 47(10): 49-54.
|
| [17] |
张勇, 张守攻, 齐力旺, 等. 杨树——林木基因组学研究的模式物种[J]. 植物学通报, 2006, 22(3): 286-293.
|
| [18] |
|
| [19] |
刘润进, 陈应龙. 菌根学[M]. 北京: 科学出版社, 2007: 1-447.
|
| [20] |
刘凯洋, 邱智军, 张巧明, 等. 丛枝菌根真菌对砷胁迫下棉花根系形态和生理特征的影响[J]. 西北植物学报, 2021, 41(7): 1188-1198.
|
| [21] |
孙兴华, 周晓榕, 庞保平, 等. 南美斑潜蝇为害对黄瓜叶片中蛋白酶抑制剂活性及葫芦素B含量的影响[J]. 应用昆虫学报, 2014, 51(1): 169-177.
|
| [22] |
|
| [23] |
|
| [24] |
徐正浩, 崔绍荣, 何勇, 等. 植物次生代谢物质和害虫防治[J]. 植物保护, 2004, 30(4): 8-11.
|
| [25] |
雒珺瑜, 崔金杰, 辛惠江. 棉花叶片纤维素和木质素含量与绿盲蝽抗性的关系[J]. 西北农林科技大学学报(自然科学版), 2012, 40(4): 81-85.
|
| [26] |
|
| [27] |
周琳, 冯俊涛, 张锦恬, 等. 雷公藤总生物碱对几种昆虫的生物活性[J]. 植物保护, 2007, 33(6): 60-64.
|
| [28] |
周北雅, 郭艳东, 薛雅鞠, 等. 功能蛋白酶催化及应用进展[J]. 生物加工过程, 2023, 21(4):419-438.
|
| [29] |
何应, 马向丽, 任健, 等. 蝗虫取食对毛花雀稗防御酶活性的影响[J]. 草业科学, 2021, 38(11): 2294-2300.
|
| [30] |
杨乃博, 伍苏然, 沈林波, 等. 植物抗虫性研究概况[J]. 热带农业科学, 2014, 34(9): 61-68,89.
|
| [31] |
|
| [32] |
宋福强, 杨国亭, 孟繁荣, 等. 丛枝菌根(AM)真菌对大青杨苗木根系的影响[J]. 南京林业大学学报(自然科学版), 2005, 29(6): 35-39.
|
| [33] |
吕敏, 卫甜, 刘怀阿, 等. 昆虫取食和机械损伤对棉花和玉米脂氧合酶活性的诱导作用[J]. 江苏农业科学, 2021, 49(10): 86-90.
|
| [34] |
陈宝玲, 杨开太, 黄森, 等. 有益菌根真菌及其互作对带叶兜兰试管苗生理生长的影响[J]. 西南林业大学学报(自然科学), 2022, 42(2): 19-25.
|
| [35] |
|
| [36] |
赵海龙. 苜蓿斑蚜胰蛋白酶抑制剂筛选及效果评价[D]. 沈阳: 沈阳农业大学, 2018.
|
| [37] |
|
| [38] |
|
| [39] |
任茂琼, 李家慧, 褚旭东, 等. 不同抗性玉米自交系对朱砂叶螨体内解毒酶活性的影响差异[J]. 中国植保导刊, 2017, 37(11): 15-18.
|
| [40] |
|
| [41] |
|
| [42] |
李定银, 郅军锐, 张涛, 等. 乙基多杀菌素和乙虫腈对西花蓟马解毒酶和乙酰胆碱酯酶活性的影响[J]. 应用昆虫学报, 2020, 57(6): 1385-1393.
|
| [43] |
王沫. 美国白蛾对植物次生代谢物质的适应性[D]. 哈尔滨: 东北林业大学, 2020.
|
| [44] |
|
/
| 〈 |
|
〉 |