 PDF(4516 KB)
PDF(4516 KB)


Structural prediction of glutathione S-transferase (GST) in Lymantria dispar and its molecular docking analysis with poplar secondary metabolites
XIE Jiaming, CAO Chuanwang, SUN Lili, LI Mingjun, ZHANG Ruiqiong
Journal of Nanjing Forestry University (Natural Sciences Edition) ›› 2024, Vol. 48 ›› Issue (5) : 211-220.
 PDF(4516 KB)
PDF(4516 KB)
 PDF(4516 KB)
PDF(4516 KB)
Structural prediction of glutathione S-transferase (GST) in Lymantria dispar and its molecular docking analysis with poplar secondary metabolites
【Objective】This study aims to determine the binding ability and mode of glutathione S-transferase (GST) in Lymantria dispar to key poplar secondary metabolites, provide a foundational theory for the adaptation mechanism of LdGST to these metabolites. Additionally, The GST molecular simulation was used to identify the best binding secondary metabolites, offering a novel strategy for controlling Lymantria dispar.【Method】Homology modeling, multiple sequence alignment, and three-dimensional structure determination of 10 GSTs were performed using templates with over 30% similarity via the Swiss-model website. The 10 GST models were evaluated using SAVES software. The 3D structures of six poplar secondary metabolites were obtained from the PubChem website. Molecular docking of the 10 GST models with the six poplar secondary metabolites was conducted using Discovery Studio 2019 Client software, and docking results analyzed through combined energy and visualization.【Result】 The models obtained through homology modeling of the 10 GSTs met the criteria, with more than 90% of amino acids in the Ramachandran Plot’s most favored and additional allowed regions. The percentage of amino acids with a compatibility score above 0.2 between the three-dimensional and primary structures was over 80%, and the ERRAT value ranged from 91.73% to 97.82%, indicating the models were qualified. Molecular docking revealed that the binding of GST to poplar secondary metabolites involved hydrogen and covalent bonds. The optimal protein bindings were as follows: Salicin, LdGSTs2 with a binding energy of -45.70 kJ/mol. Caffeic acid, LdGSTz2 with a binding energy of -43.96 kJ/mol. Catechol and rutin, LdGSTz1 with binding energies of -25.86 and -95.46 kJ/mol, respectively. Flavonoids, LdGSTe2 with a binding energy of -32.49 kJ/mol. Quercetin, LdGSTo2 with a binding energy of -62.09 kJ/mol.【Conclusion】The binding energy of LdGSTs to poplar secondary metabolites are all below -5 kJ/mol, involving hydrogen and covalent bonds. The similar binding energy of the same poplar secondary metabolites to different GSTs suggests good affinity and stable intermolecular binding, with low specificity of GST for secondary metabolites. However, the affinity of the same GST to different poplar secondary metabolites varied. These results provide a theoretical basis for reducing insecticide resistance by incorporating secondary metabolites.
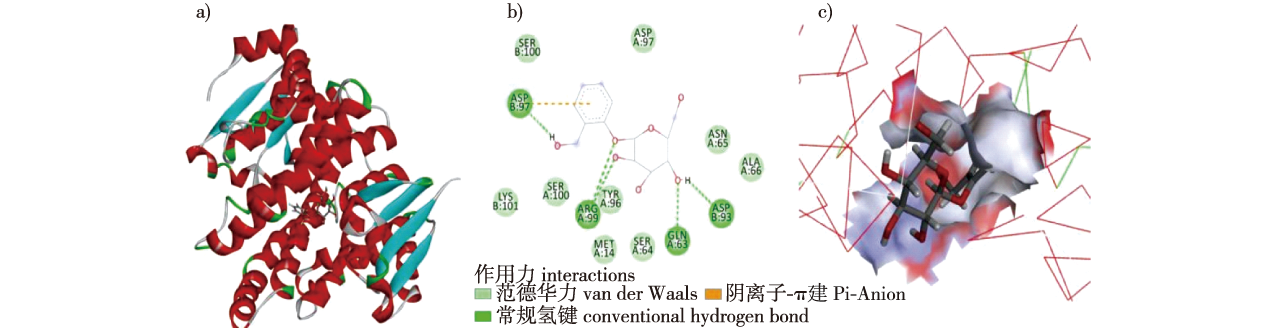
Lymantria dispar / glutathione S-transferase(GST) / poplar secondary metabolites / homology modeling / molecular docking / binding energy
| [1] |
郭冰, 郝恩华, 王菁桢, 等. 入侵害虫松树蜂气味结合蛋白与其相关信息化学物质的分子对接[J]. 植物保护学报, 2019, 46(5):1004-1017.
|
| [2] |
李敏, 郭美琪, 相伟芳, 等. 分子对接技术在昆虫化学感受研究中的应用进展[J]. 植物保护, 2019, 45(5):121-127.
|
| [3] |
李红亮, 张林雅, 庄树林, 等. 中华蜜蜂普通气味结合蛋白ASP2的气味结合功能模式分析[J]. 中国农业科学, 2013, 46(1):154-161.
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
张龙. 飞蝗嗅觉的细胞与分子机制研究进展[J]. 生命科学, 2010, 22(12):1215-1228.
|
| [14] |
|
| [15] |
杨雪清, 刘吉元, 张雅林. 分子模拟技术及其在苹果蠹蛾代谢杀虫剂分子机制研究中的应用进展[J]. 生物安全学报, 2015, 24(4):265-273.
|
| [16] |
张元. 氟虫腈与昆虫GABA受体相互作用的研究[D]. 上海: 上海师范大学, 2016.
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
周郑, 程新胜, 王方晓, 等. 烟碱和芸香苷对斜纹夜蛾药剂敏感性及相关酶活性的影响[J]. 农药学学报, 2007, 9(3):305-308.
|
| [22] |
高希武, 董向丽, 郑炳宗, 等. 棉铃虫的谷胱甘肽S-转移酶(GSTs):杀虫药剂和植物次生性物质的诱导与GSTs对杀虫药剂的代谢[J]. 昆虫学报, 1997, 40(2):122-127.
|
| [23] |
胡春祥. 舞毒蛾生物防治研究进展[J]. 东北林业大学学报, 2002, 30(4):40-43.
|
| [24] |
王亚军, 邹传山, 王若茜, 等. 3种植物次生代谢物质对舞毒蛾的杀虫活性分析[J]. 北京林业大学学报, 2017, 39(11):75-81.
|
| [25] |
鄢杰明, 廖月枝, 严善春, 等. 甲氧虫酰肼对舞毒蛾解毒酶和保护酶活性的影响[J]. 东北林业大学学报, 2010, 38(11):112-114.
|
| [26] |
鄢杰明, 钟华, 严俊鑫, 等. 多杀菌素对舞毒蛾幼虫解毒酶活性的影响[J]. 林业科学, 2012, 48(9):82-87.
|
| [27] |
冯春富, 严善春, 鲁艺芳, 等. 兴安落叶松诱导抗性对舞毒蛾幼虫解毒酶活性的影响[J]. 林业科学, 2011, 47(8):102-107.
|
| [28] |
|
| [29] |
吕春鹤, 张国财, 邹传山. 白屈菜总碱对舞毒蛾离体酶活性的影响[J]. 中国林副特产, 2017(4):33-36,38.
|
| [30] |
王振越. 杨树主要次生物质对舞毒蛾生长发育及主要解毒酶影响[D]. 哈尔滨: 东北林业大学, 2020.
|
| [31] |
许力山. 三种次生物质与溴氰虫酰胺对舞毒蛾P450和GST影响研究[D]. 哈尔滨: 东北林业大学, 2021.
|
| [32] |
杨欢, 郭冰, 郝恩华, 等. 禾谷缢管蚜气味降解酶鉴定及其与关键信息化学物质的分子对接[J]. 植物保护学报, 2022, 49(4):1119-1131.
|
| [33] |
|
| [34] |
崔琳琳, 宋亚刚, 苗明三. 基于网络药理学和分子对接的陈皮干预COVID-19的可能机制[J]. 中药药理与临床, 2020, 36(5):28-33.
|
| [35] |
马天翔, 顾志荣, 孙岚萍, 等. 荆芥-防风药对治疗荨麻疹作用机制的网络药理学研究[J]. 中药新药与临床药理, 2020, 31(4):435-440.
|
| [36] |
|
| [37] |
|
| [38] |
虞唯道, 刘淼, 宋萍, 等. 鞣花酸的生物活性与分析研究进展[J]. 生物加工过程, 2023, 21(1):83-90.
|
| [39] |
史宗畔, 冉永红, 张晶晶, 等. 中华按蚊气味结合蛋白AsinOBP1与避蚊胺(DEET)的结合特性分析[J]. 昆虫学报, 2018, 61(1):139-148.
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
詹丽, 李敬丹, 付璇, 等. 紫茉莉种子中对草地贪夜蛾的杀虫活性成分及杀虫机制[J]. 江苏农业学报, 2024, 40(1):47-54.
|
/
| 〈 |
|
〉 |