 PDF(3320 KB)
PDF(3320 KB)


Study on leaf color parameters, pigment content and anatomical structure of Triadica cochinchinensis families
HU Yanping, LIU Weidong, ZHANG Min, CHEN Minggao, CHENG Yong, WEI Zhiheng, PANG Wensheng, WU Jiyou
Journal of Nanjing Forestry University (Natural Sciences Edition) ›› 2025, Vol. 49 ›› Issue (2) : 123-133.
 PDF(3320 KB)
PDF(3320 KB)
 PDF(3320 KB)
PDF(3320 KB)
Study on leaf color parameters, pigment content and anatomical structure of Triadica cochinchinensis families
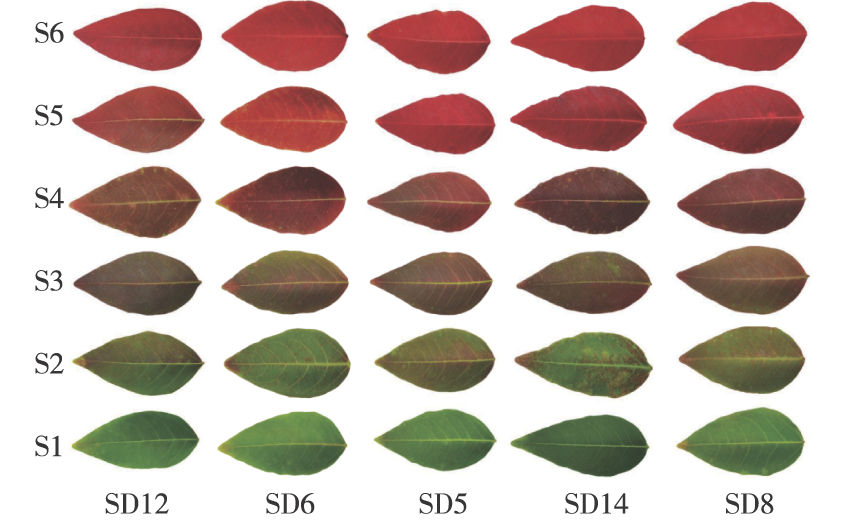
【Objective】This study aims to explore the changes in leaf pigment content and color response of different genetic materials over various periods and to analyze the key factors influencing leaf coloration in five families of Triadica cochinchinensis from physiological and tissue structural perspectives. The findings are expected to provide a theoretical basis for selecting superior landscape varieties of T. cochinchinensis.【Method】 Five families of T. cochinchinensis with a height of 50 cm were selected as research materials. The leaf color changes of six time points (S1. 2021-10-29; S2. 2021-11-05; S3.2021-11-12; S4. 2021-11-19; S5. 2021-11-26; S6: 2021-12-03) were observed under natural conditions. Leaf color parameters (L*, a*, b*), pigment content (chlorophyll, carotene, anthocyanin), and anatomical structure (upper and lower epidermis, palisade tissue, spongy tissue) were measured regularly. The dynamic changes in leaf color and genealogy selection of T. cochinchinensis were analyzed.【Result】(1)The leaf color of each T. cochinchinensis genealogy during the S1—S6 period primarily transitioned from light green to dark brownish yellow, then to reddish brown, and finally to bright red, with the ornamental period concentrated in the S5—S6 period. Among them, the SD8 family exhibited the highest ornamental value, with a color-changing period from the onset of discoloration (S2) to complete defoliation lasting 37 days.(2)The leaf color parameter a* value of each family showed a linear upward trend, while the L* and b* values exhibited different change patterns. Specifically, greener leaves corresponded to lower a* values, whereas redder leaves corresponded to higher a* values, making a* a representative parameter for the reddish leaf color of the five families.(3)Throughout the entire cycle, the chlorophyll and carotenoid contents of all T. cochinchinensis families decreased, while anthocyanin content increased, reaching a maximum at S3. The change from green to red leaves was mainly due to the proportionate distribution of pigment content. Before S3, chlorophyll content dominated the green leaf stage, and after S3, anthocyanin content dominated the red leaf stage.(4)During the color change from green to red, leaf thickness measurements indicated that pre-color change thickness was greater than post-color change thickness. The SD4 family experienced a significant 29.17% decrease in leaf thickness compared to other families (P<0.05). The upper epidermis thickness was greater than the lower epidermis across all families. Before the color change, the upper epidermis thickness of the SD6 family was significantly smaller than that of other families (P<0.05). Post-color change, the combined upper and lower epidermis thickness of the SD6 family accounted for the largest proportion among all families, significantly differing from others (P<0.05). Spongy tissue thickness exceeded palisade tissue thickness. The SD4 and SD6 families exhibited the most significant changes in spongy tissue thickness before and after the color change, at 30.59% and 0.87%, respectively. Palisade tissue thickness in the SD8 family significantly differed from other families (P<0.05), with a maximum change range of 29.92%.(5)Post-veraison, the leaf tissue structure of most T. cochinchinensis families showed that decreased leaf thickness, palisade tissue, and spongy tissue thickness facilitated anthocyanin accumulation, turning leaves red. No obvious variation attachments, such as particles or wax, were observed in leaf tissue, and no large air chambers appeared.【Conclusion】Leaf color change in T. cochinchinensis is primarily influenced by pigment dynamics, with anthocyanins being the main factor causing leaf reddening. Among the five families studied, the SD8 family demonstrated the most outstanding leaf color change and high ornamental value, making it particularly suitable for landscape greening. This study provides robust theoretical support for selecting T. cochinchinensis varieties and evaluating their ornamental value.
Triadica cochinchinensis families / leaf color change / leaf color parameter / pigment content / anatomical structure
| [1] |
缴丽莉, 路斌, 翟士勇, 等. 石家庄市彩叶树种资源和物候观赏特征研究[J]. 西北林学院学报, 2015, 30(04): 283-288.
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
郭欢欢, 刘勇, 姚飞, 等. 不同种源黄连木秋季色素含量与叶色参数的关系[J]. 西北植物学报, 2017, 37(10): 2003-2009.
|
| [7] |
冯新康, 喻春明, 朱爱国, 等. 不同苎麻品种叶片色素含量与叶色参数的关系[J]. 中国麻业科学, 2021, 43(06): 310-315.
|
| [8] |
王振兴, 于云飞, 陈丽, 等. 彩叶植物叶片色素组成、结构以及光合特性的研究进展[J]. 植物生理学报, 2016, 52(1):1-7.
|
| [9] |
梁玲, 黄玉琼, 陈小红. 不同色彩珙桐叶片和苞片解剖结构及色素含量比较研究[J]. 西北植物学报, 2020, 40(9):1539-1548.
|
| [10] |
孙旺旺, 孟宪敏, 徐秀源, 等. 金叶连翘叶片色素含量和解剖结构研究[J]. 植物研究, 2020, 40(3):321-329.
|
| [11] |
史莹, 张丽珍, 曾志将, 等. 山乌桕蜂蜜酒酿造酵母的筛选、鉴定及应用[J]. 中国食品学报, 2013, 13(10):197-204.
|
| [12] |
|
| [13] |
|
| [14] |
杨明友, 吴际友, 程勇, 等. 山乌桕富根壮苗培育试验[J]. 湖南林业科技, 2020, 47(5):68-71.
|
| [15] |
黄朝法. 山乌桕优良家系幼林期生长与观赏性分析及选择[J]. 南方林业科学, 2019, 47(5):17-19,74.
|
| [16] |
林锦森. 山乌桕的特征特性及育苗造林技术[J]. 现代农业科技, 2011(8):193-193,195.
|
| [17] |
齐睿, 李小红, 石博雨, 等. 红叶石楠转色期叶片色彩参数与色素含量的相关性分析[J]. 河南农业科学, 2019, 48(4):93-101.
|
| [18] |
唐前瑞, 陈德富, 陈友云, 等. 红檵木叶色变化的生理生化研究[J]. 林业科学, 2006, 42(2):111-115.
|
| [19] |
廖文婷, 王瑞辉, 钟飞霞, 等. 5个油茶优良无性系光合及叶片解剖特征比较分析[J]. 经济林研究, 2015, 33(1):56-61.
|
| [20] |
于杨, 姚程程, 张洁, 等. 吉林省两城市常见绿化植物单体色彩美景度评价[J]. 北华大学学报(自然科学版), 2017, 18(4):521-527.
|
| [21] |
李力, 张盛楠, 刘亚敏, 等. 基于Lab模型的北美红枫呈色生理因素探究[J]. 西北农林科技大学学报(自然科学版), 2017, 45(9):87-94.
|
| [22] |
杨淑红, 朱延林, 马永涛, 等. 生长季全红杨叶色与色素组成的相关性[J]. 东北林业大学学报, 2013, 41(7):63-68.
|
| [23] |
郭卫珍, 李湘鹏, 宋垚, 等. 5个山茶品种冬季叶片色素含量和叶色参数的变化初探[J]. 江西农业大学学报, 2018, 40(3):508-515.
|
| [24] |
苏金, 黄婧, 周鹏, 等. 黄金枸骨变色期的叶色与叶片光合色素含量关系研究[J]. 江苏林业科技, 2019, 46(4):33-36.
|
| [25] |
沈星诚, 周婷, 范俊俊, 等. 日本红枫春季叶片色彩评价[J]. 南京林业大学学报(自然科学版), 2020, 44(6):213-220.
|
| [26] |
姜卫兵, 庄猛, 韩浩章, 等. 彩叶植物呈色机理及光合特性研究进展[J]. 园艺学报, 2005, 32(2):352-358.
|
| [27] |
吴琰琰, 刘玉民, 闫洋洋. 北美红枫秋季呈色的生理机制研究[J]. 西南大学学报(自然科学版), 2019, 41(5):21-29.
|
| [28] |
韩培培. 3种杉科植物秋季叶色变化的生理生化研究[D]. 杭州: 浙江农林大学, 2014.
|
| [29] |
唐东芹, 徐怡倩, 袁媛, 等. 理化因素对风信子花色苷稳定性的影响[J]. 南京林业大学学报(自然科学版), 2016, 40(4):69-73.
|
| [30] |
仲磊, 张焕朝, 范俊俊, 等. 夏季淹水胁迫对北美枫香苗木叶色及光合荧光特性的影响[J]. 南京林业大学学报(自然科学版), 2021, 45(2):69-76.
|
| [31] |
徐展宏, 朱莹, 金慧颖, 等. 不同叶色青钱柳叶片色素、多酚含量及光合特性的差异[J]. 南京林业大学学报(自然科学版), 2022, 46(2):103-110.
|
| [32] |
路买林, 陈梦娇, 张嘉嘉, 等. '红叶'杜仲叶色转变过程中叶片生理指标变化[J]. 南京林业大学学报(自然科学版), 2021, 45(1):86-92.
|
| [33] |
王振兴, 曹建冉, 秦红艳, 等. 狗枣猕猴桃彩叶色素含量和结构共同影响叶色[J]. 植物生理学报, 2016, 52(12):1921-1926.
|
| [34] |
季鹏章, 梁名志, 宋维希, 等. 茶树珍稀品种“紫娟” 的叶片色素含量与叶色变化的关系研究[J]. 西南农业学报, 2010, 23(6):1860-1862.
|
| [35] |
刘雪梅, 金晓玲, 汪晓丽, 等. 3类色系榉树叶色表达期色素含量变化规律研究[J]. 河南农业大学学报, 2014, 48(5):596-601.
|
| [36] |
黄可, 王小德, 柳翼飞, 等. 红枫春季叶色变化与色素含量的相关性[J]. 浙江农林大学学报, 2012, 29(5):734-738.
|
| [37] |
崔祺, 吴昀, 李东泽, 等. 彩叶桂叶片发育过程中叶色表型与色素成分变化[J]. 南京林业大学学报(自然科学版), 2023, 47(2):79-86.
|
| [38] |
陈小红, 徐扬, 刘韩, 等. 川西高原4种高山海棠的根茎解剖结构特征及其抗旱响应策略分析[J]. 西北植物学报, 2017, 37(7):1296-1302.
|
| [39] |
罗玉兰, 张国兵, 张爱明. ‘金边’北美鹅掌楸不同叶色结构特征比较[J]. 植物研究, 2013, 33(3):282-286.
|
| [40] |
张少露. 红叶石楠叶色变化过程中叶结构和生理特征研究[D]. 雅安: 四川农业大学, 2017.
|
| [41] |
尹国平, 刘雄盛, 蒋燚, 等. 枫香变红过程中叶片组织结构、光合特性及色素含量变化研究[J]. 广西植物, 2022, 42(7):1213-1221.
|
| [42] |
袁明, 万兴智, 杜蕾, 等. 红花檵木叶色变化机理的初步研究[J]. 园艺学报, 2010, 37(6):949-956.
|
| [43] |
|
| [44] |
王萌, 常格, 王琦, 等. 4种园林树木叶片秋季变色期的呈色机理[J]. 林业与生态科学, 2020, 35(1):93-98.
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
吴涛, 耿云芬, 柴勇, 等. 三叶爬山虎叶片解剖结构和光合生理特性对3种生境的响应[J]. 生态环境学报, 2014, 23(10):1586-1592.
|
| [49] |
孙旺旺. 金叶金钟花叶片色素含量年变化及解剖结构特征[D]. 秦皇岛: 河北科技师范学院, 2018.
|
| [50] |
苏佳露, 史无双, 杨雅运, 等. 6个竹种叶色与光合色素含量及叶片结构比较[J]. 林业科学, 2020, 56(7):194-203.
|
/
| 〈 |
|
〉 |