 PDF(3179 KB)
PDF(3179 KB)


Sex dimorphism of the adults Xanthogaleruca aenescens antennal sensilla
TAO Mengmeng, MENG Zhaojun, YAN Shanchun, LYU Jinyan, ZHANG Chunwen, YE Yi, WU Chengdan
Journal of Nanjing Forestry University (Natural Sciences Edition) ›› 2025, Vol. 49 ›› Issue (3) : 197-204.
 PDF(3179 KB)
PDF(3179 KB)
 PDF(3179 KB)
PDF(3179 KB)
Sex dimorphism of the adults Xanthogaleruca aenescens antennal sensilla
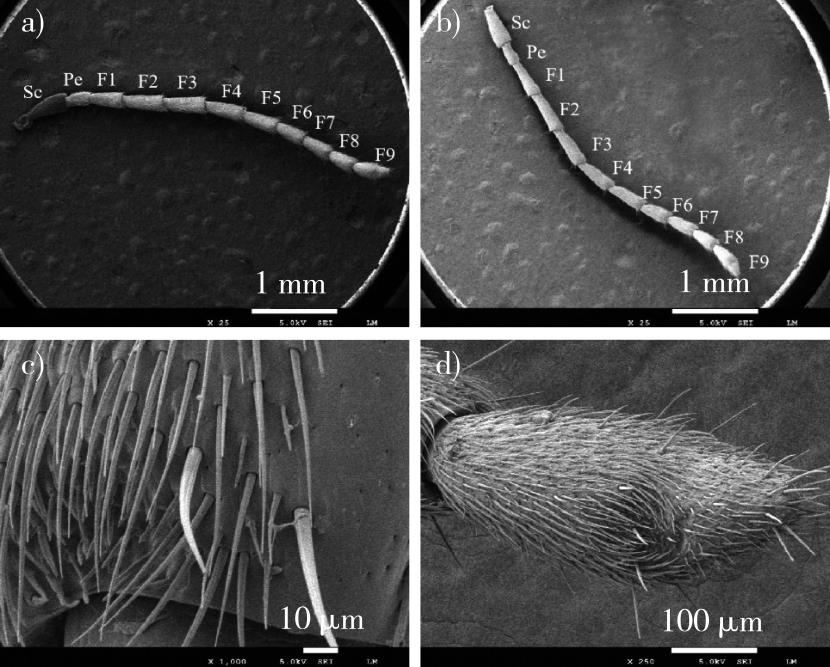
【Objective】This study aimed to identify the types, distribution, quantity, and sexual dimorphism of antennal sensilla in Xanthogaleruca aenescens through ultrastructural observation. The findings provide a basis for understanding the genetic evolution, morphological classification, and the role of olfactory sensilla in host volatile detection in leaf beetles.【Method】The antennae of adult female and male X. aenescens were cleaned, air-dried, and gold-coated for scanning electron microscopy (SEM). SEM was used to observe and photograph the morphological characteristics of the antennae and sensilla of both sexes, as well as to identify the types of sensilla present. Independent sample t-tests were conducted to compare differences in antennal length, sensilla length, base diameter, and the number of sensilla between the sexes, and the distribution of sensilla across the antennae was also examined.【Result】The antennae of X. aenescens were linear and consist of the scape, pedicel, and nine flagellar subsegments. The average length of male antennae was (4 558.01 ± 104.15) μm, while that of females was (4 488.75 ± 224.33) μm. Males have slightly longer antennae than females, although this difference was not statistically significant. Both males and females possessed eight types of antennal sensilla: sensilla trichodea (St), sensilla chaetica (Sc), sensilla basiconica (Sb), sensilla styloconica (Sst), Böhm bristles (Bb), sensilla gemmiformium (Sg), sensilla coeloconica (Sco), and sensilla auricillica (Sau). St and Sb each have four subtypes, Sc has two subtypes, and Sst has three subtypes. The female antennal flagellum has Sst 2, while the male flagellum contains Sst 3. The most abundant sensilla are St, followed by Sb, while Sco is the least common. St 4, Sb 1, Sb 3, and Sau exhibit micropores on their surfaces, indicating their role as olfactory sensilla. The total number of antennal sensilla in females was significantly greater than in males (P < 0.01). Specifically, the numbers of St 2, Sb 2, and Sst 1 were significantly higher in females (P < 0.05). St 4 and Sb 3 is significantly higher in females than in males (P < 0.01), while Sau was significantly fewer in females compared to males (P < 0.01). No significant differences were observed in the number of other sensilla. In both sexes, the tips of antennal subsegments F2 to F8 had inwardly concave areas with a high density of sensilla, and a similar concave area was present at the middle of the terminal segment F9. The density and number of sensilla increased from the base to the tip of the antenna. Additionally, the ventral surface of the male antennal flagellum (F1 to F8) exhibited a more densely packed area of epidermal depressions, a feature absent in females.【Conclusion】Both female and male X. aenescens have the same types of antennal sensilla, but there are clear sexual dimorphisms in sensilla subtypes, quantity, and distribution. Different subtypes of Sst were observed in males and females, with variations in the size of sensilla between the sexes. The total number of sensilla in males was significantly lower than in females. The specific roles of these sensilla in environmental perception, host detection, and mating behaviors should be further explored using transmission electron microscopy, electrophysiology, and other advanced techniques.
Xanthogaleruca aenescens / antennal sensilla / scanning electron microscopy / sex dimorphism / antennal morphology / ultrastructure
| [1] |
李成德. 森林昆虫学[M]. 2版. 北京: 中国林业出版社, 2022.
|
| [2] |
马瑞燕, 杜家纬. 昆虫的触角感器[J]. 昆虫知识, 2000, 37(3):179-183.
|
| [3] |
段云博, 朱晓珍, 叶家桐, 等. 红棕象甲触角感器的超微结构[J]. 植物保护, 2020, 46(6):103-110.
|
| [4] |
张聪, 王振营, 何康来, 等. 双斑长跗萤叶甲触角感器的扫描电镜观察[J]. 应用昆虫学报, 2012, 49(3):756-761.
|
| [5] |
王桂荣, 郭予元, 吴孔明. 棉铃虫触角感器的超微结构观察[J]. 中国农业科学, 2002, 35(12):1479-1482,1584-1586.
|
| [6] |
|
| [7] |
刘丽玲, 李海滨, 管维, 等. 马铃薯甲虫成虫触角及感器的扫描电镜观察[J]. 植物检疫, 2021, 35(2):20-23.
|
| [8] |
徐伟, 樊瑞冬, 伍名渊, 等. 斑鞘豆叶甲触角感器的超微结构[J]. 环境昆虫学报, 2020, 42(1):227-236.
|
| [9] |
邢雪. 榆紫叶甲触角感器及其功能化合物的筛选研究[D]. 长春: 东北师范大学, 2016.
|
| [10] |
|
| [11] |
|
| [12] |
张晓军, 孙伟, 张健, 等. 鞘翅目昆虫触角感器研究进展[J]. 安徽农业科学, 2013, 41(7):2932-2935.
|
| [13] |
兰晓娜, 向姗姗, 朱慧. 昆虫触角感器类型及其功能研究进展[J]. 环境昆虫学报: 2023,1-27.
|
| [14] |
王涛. 红脂大小蠹成虫触角感器的扫描电镜观察[J]. 植物保护, 2017, 43(2):112-116,128.
|
| [15] |
|
| [16] |
张锋, 洪波, 王远征, 等. 枣食芽象甲触角感器的扫描电镜观察[J]. 西北农业学报, 2019, 28(8):1373-1379.
|
| [17] |
徐存翊, 王昊, 叶茂翔, 等. 油茶象触角感器的扫描电镜观察[J]. 植物保护, 2021, 47(3):101-108.
|
| [18] |
|
| [19] |
张新民, 赵宁, 韩秀, 等. 核桃长足象触角感器的扫描电镜观察[J]. 生物学杂志, 2021, 38(4):96-99.
|
| [20] |
刘晓梅, 凌冰, 赵旭伟, 等. 黄曲条跳甲触角感器的扫描电镜观察[J]. 环境昆虫学报, 2015, 37(1):68-76.
|
| [21] |
|
| [22] |
江望锦, 嵇保中, 刘曙雯, 等. 天牛成虫信息素及嗅觉感受机制研究进展[J]. 昆虫学报, 2005, 48(3):427-436.
|
| [23] |
尚军烨, 徐炜超, 孟庆繁, 等. 栎丽虎天牛成虫触角感器的扫描电镜观察[J]. 南京林业大学学报(自然科学版), 2021, 45(5):195-200.
|
/
| 〈 |
|
〉 |