 PDF(2917 KB)
PDF(2917 KB)


Preliminary study on the function of Gymnosporangium yamadae effector GyHGSRE1
GAO Xinmei, SHAO Chenxi, LIANG Yingmei, LAO Wenhao
Journal of Nanjing Forestry University (Natural Sciences Edition) ›› 2025, Vol. 49 ›› Issue (5) : 209-216.
 PDF(2917 KB)
PDF(2917 KB)
 PDF(2917 KB)
PDF(2917 KB)
Preliminary study on the function of Gymnosporangium yamadae effector GyHGSRE1
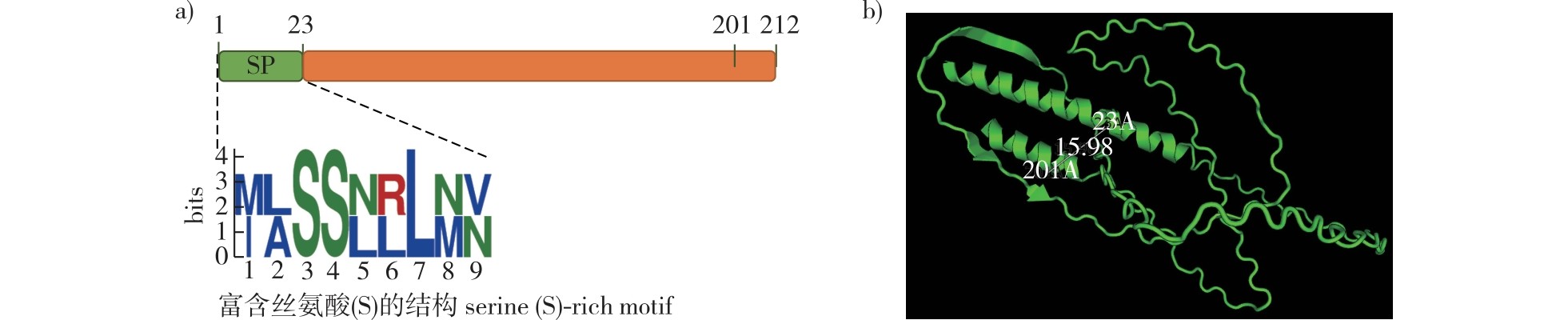
【Objective】The study determined the core biological function of the effector protein GyHGSRE1 secreted by the Gymnosporangium yamadae haustoria. This work provides foundational data for elucidating the molecular mechanisms of G. yamadae effector protein.【Method】The haustorial transcriptome of G. yamadae prioritized GyHGSRE1 as a highly expressed effector (FPKM = 117.92). MEME (http://meme-suite.org/) predicted its two-dimensional structure, while Tencent AI (https://drug.ai.tencent.com) generated the three-dimensional model. Real-time PCR quantified GyHGSRE1 expression level during fungal infection. Yeast secretion assays validated the signal peptide’s secretory capacity. Transient expression via Agrobacterium tumefaciens assessed GyHGSRE1 function in Nicotiana benthamiana and apple (Malus domestica) leaves.【Result】GyHGSRE1 contains an N-terminal serine-rich signal peptide. qRT-PCR demonstrated peak expression during haustorium maturation and spore development (pycniosporophores/aeciospores). The protein was localized to plant cell cytoplasm and nuclei, triggering cell death and immune responses in leaf cells of N. benthamiana. Full-length GyHGSRE1 induced cell death in apple leaves, but deletion of the signal peptide attenuated this activity.【Conclusion】The glycine-serine-rich atypical effector GyHGSRE1 exhibits dual localization and cell necrosis-inducing effects across plant species, implying broad-spectrum elicitor potential. Its expression correlates with critical infection stages (host colonization and sporulation). The signal peptide may mediate functional specificity, likely influencing host-pathogen recognition.
Gymnosporangium yamadae / atypical effector protein / elicitor / signal peptide / plant immunity
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
刘霞, 陶思齐, 翁涵, 等. 山田胶锈菌和亚洲胶锈菌吸器提取体系建立[J]. 菌物学报, 2019, 38(9): 1430-1439.
|
| [8] |
翁涵, 刘霞, 陶思齐, 等. 山田胶锈菌和亚洲胶锈菌吸器的比较转录组分析[J]. 生物工程学报, 2022, 38(10): 3825-3843.
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
赵薇, 许彤骏, 王喻元, 等. 溶藻细菌的筛选及群体感应信号对其活力的调节作用[J]. 生物加工过程, 2023, 21(4):461-470.
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
/
| 〈 |
|
〉 |