 PDF(2763 KB)
PDF(2763 KB)


Variation and trade-offs of twig and leaf traits among different broadleaved life form plants in the primitive broadleaved-Korean pine forest
HOU Xuanzhu, LI Nan
Journal of Nanjing Forestry University (Natural Sciences Edition) ›› 2025, Vol. 49 ›› Issue (3) : 163-171.
 PDF(2763 KB)
PDF(2763 KB)
 PDF(2763 KB)
PDF(2763 KB)
Variation and trade-offs of twig and leaf traits among different broadleaved life form plants in the primitive broadleaved-Korean pine forest
【Objective】The trade-off between twig and leaf traits is crucial for understanding how plants allocate resources under various environmental stresses. This study focused on 12 dominant or common broadleaved plants in a natural broadleaved-Korean pine (Pinus koraiensis) forest. 【Method】We used one-way ANOVA or non-parametric rank-sum tests to analyze trait differences among different life forms or species. Standardized major axis estimation (SMA) was employed to investigate correlations between twig and leaf traits. 【Result】Broadleaved plants of different life forms exhibited similar trends in twig and leaf traits. Specifically, variation in twig cross-sectional area and volume-based leafing intensity were greater than 30%, twig mass variation exceeded 20%, while individual leaf mass, total leaf area, and individual leaf area variations were below 15%. Trees had significantly higher individual leaf area, total leaf area, and individual leaf mass compared to shrubs. There was a positive allometric relationship between twig cross-sectional area and individual leaf area in trees, while shrubs showed a positive isometric relationship. Both trees and shrubs exhibited positive isometric relationships between twig cross-sectional area and total leaf area, with only some species showing positive allometric relationships. Both tree and shrub species demonstrated negative allometric relationships between volume-based leafing intensity and individual leaf area or mass, with some tree species showing negative isometric relationships. 【Conclusion】The results suggest that twig and leaf traits across different plant life forms generally follow allometric relationships. Allometric growth enhances plant survival strategies, and the trade-off model of twig and leaf traits is influenced by growth patterns.
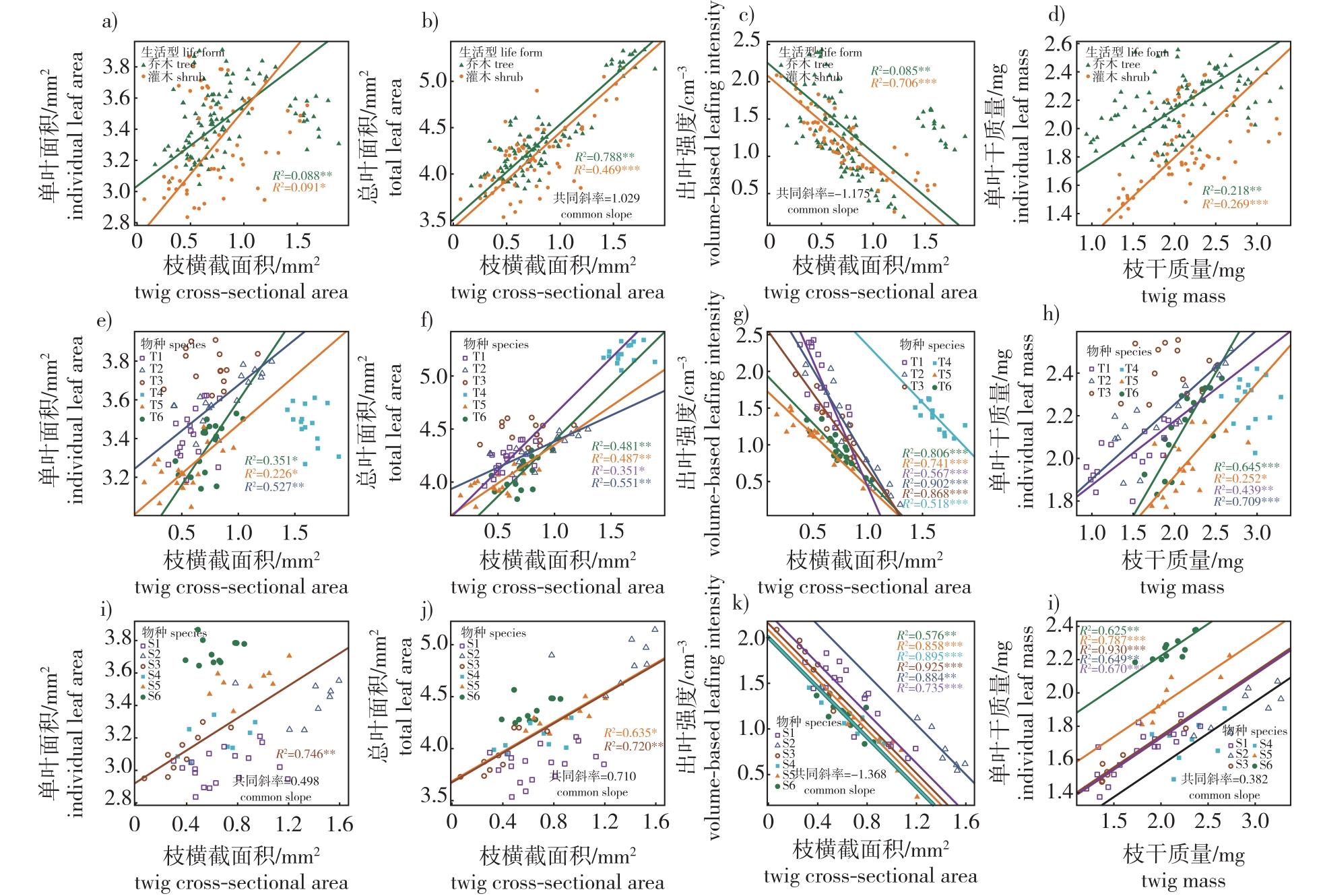
broad leaved-Korean pine forest / trade-off relationship / life form / trait variation / volume-based leafing intensity / functional traits
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
杨冬梅, 占峰, 张宏伟. 清凉峰不同海拔木本植物小枝内叶大小-数量权衡关系[J]. 植物生态学报, 2012, 36(4):281-291.
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
徐丽娜, 金光泽. 小兴安岭凉水典型阔叶红松林动态监测样地:物种组成与群落结构[J]. 生物多样性, 2012, 20(4):470-481.
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
王进, 朱江, 艾训儒, 等. 湖北星斗山地形变化对不同生活型植物叶功能性状的影响[J]. 植物生态学报, 2019, 43(5):447-457.
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
李曼, 郑媛, 郭英荣, 等. 武夷山不同海拔黄山松枝叶大小关系[J]. 应用生态学报, 2017, 28(2):537-544.
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
李锦隆, 王满堂, 李涵诗, 等. 冠层高度对江西69种阔叶树小枝单叶生物量与出叶强度关系的影响[J]. 林业科学, 2021, 57(2):62-71.
|
| [46] |
|
| [47] |
|
| [48] |
|
黑龙江凉水国家级自然保护区管理局顾伟博士在样品采集过程中给予的大力支持。
/
| 〈 |
|
〉 |