 PDF(8448 KB)
PDF(8448 KB)


Somatic embryogenesis of the new hybrid between Pinus elliottii and P. caribaea induced from immuture embryos
LI Fengqing, CHEN Jinhui, SHI Jisen
Journal of Nanjing Forestry University (Natural Sciences Edition) ›› 2026, Vol. 50 ›› Issue (1) : 23-32.
 PDF(8448 KB)
PDF(8448 KB)
 PDF(8448 KB)
PDF(8448 KB)
Somatic embryogenesis of the new hybrid between Pinus elliottii and P. caribaea induced from immuture embryos
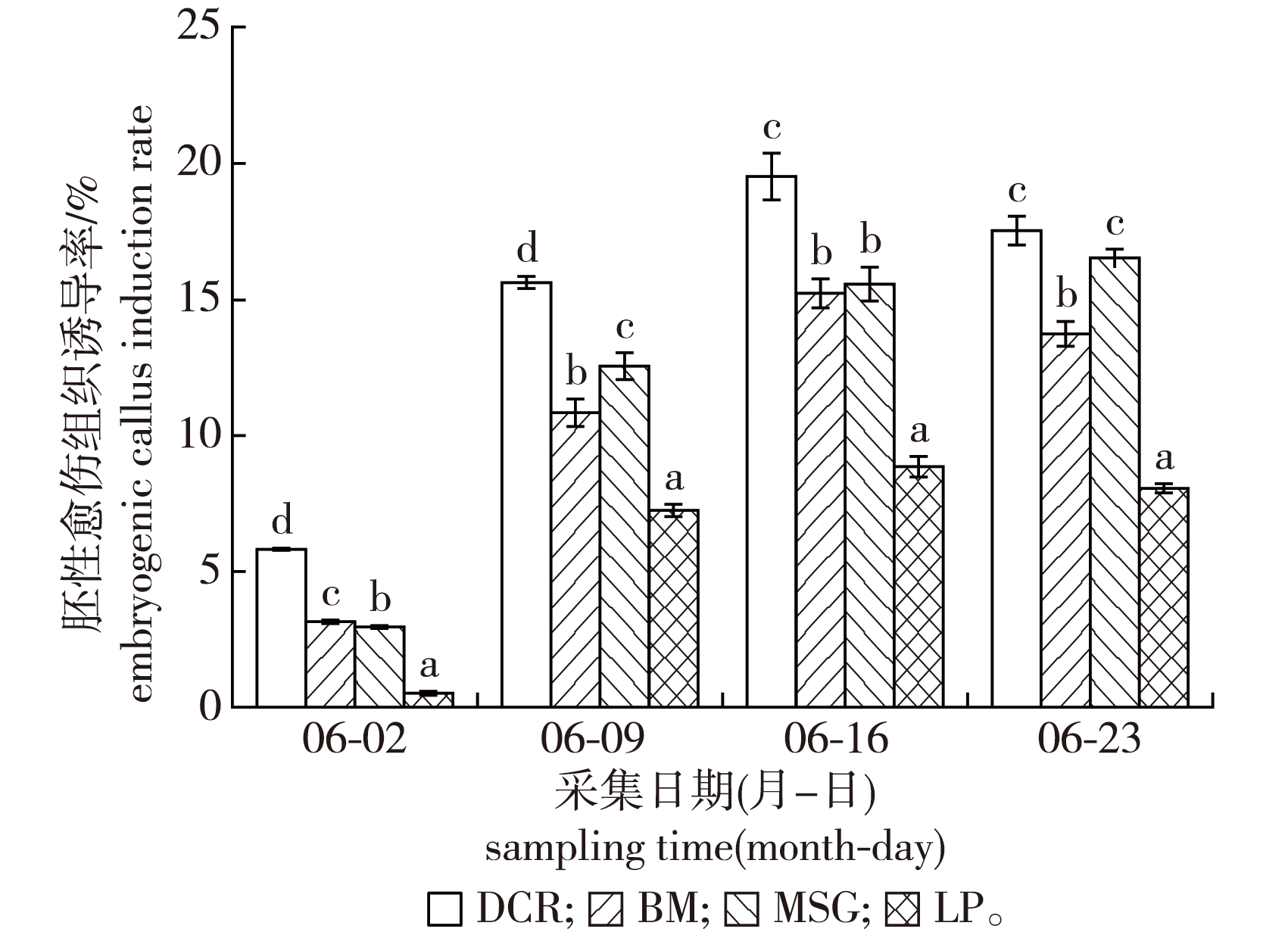
【Objective】 The large-scale production and application of the new Pinus elliottii × P. caribaea hybrid requires the application of an efficient somatic embryogenesis system. Immature zygotic embryos were used as explants to investigate which key factors influence somatic embryogenesis during embryogenic callus induction, proliferation, maturation, germination and plantlet acclimatization,establishing a comprehensive protocol for efficient SE propagation of this economically important hybrid.【Method】A total of four representative genotyes of Pinus elliottii × P. caribea were used as starting material to investigate the main factors that affect the induction of embryogenic callus: including genotype, zygotic embryo developmental stage (June 2nd to 23 rd), basic culture medium(DCR, LP, MSG and BM), hormone type and concentration, etc. Well developing embryogenic callus was analyzed for somatic embryo maturation, germination and plant regeneration. Ultimately, regenerated plants were obtained.【Result】All investigated factors (genotype, developmental stage, basic culture medium, hormone (PGR) combination and concentration) were found to have significant effects on embryogenic callus induction. The optimal time to collect explants was from June 9th to 23rd: the multi-embryo division to early cotyledon stage. The optimal embryogenic callus induction medium is DCR with 2.0 mg/L 2,4-D,1.0 mg/L BA and 0.5 mg/L KT. The average induction rate was 19.51%. To maintain the embryonic callus in a proliferative state, capable of differentiation, for a prolonged amount of time, a solid-liquid alternating culture protocol and reduced hormone concentration in the proliferation medium was required. The optimal proliferation medium was modified P6, supplemented with 0.8 mg/L 2,4-D, 0.5 mg/L BA and 0.5 mg/L KT on the solid culture medium and in the first-time liquid culture medium. During the subsequent liquid culture process, the hormone concentration should be gradually reduced. The optimal culture media for somatic embryo maturation was modified P6, with 10.0 mg/L ABA, 10.0 mg/L GA, 0.2 mg/L 2,4-D, 4 000 mg/L inositol and 2.0 mg/L activated carbon(AC). 298 mature somatic embryos were induced per mL of packed cell volume (PCV) embryogenic callus at the maturation culture cycle (6-7 weeks). Drying treatment inhibited the germination of somatic embryos, and the germination rate was best when cultured on 1/2 DCR, supplemented with 0.2 mg/L NAA, 0.5 mg/L BA and 2.0 mg/L activated carbon. Successfully germinating somatic embryos were transferred to the following medium for further cultivation until they reached 3-4 cm in height with true leaves: 1/2 DCR + 0.1 mg/L NAA+ 0.5 mg/L BA +2.5 g/L AC. Finally, the matured plantlets were transplanted and hardened onto a volume ratio of 1∶1∶1 of river sand∶perlite∶peat soil substrate and grown into robust 7-8 cm tall plants over the course of one month. 【Conclusion】In this study a complete and efficient somatic embryogenesis system for the P. elliottii × P. caribaea hybrid has been established. Furthermore, a more efficient method for embryogenic callus maintenance, using solid-liquid-solid alternating culture, has been developed. This system provides a foundational platform for large-scale clonal propagation of hybrid, with potential applications in forestation, genetic conservation, and bioreactor-based propagation systems.
Pinus elliottii × P. caribaea hybrid / embryogenic callus / somatic embryogenesis / plantlet regeneration
| [1] |
郝兆东, 马筱筱, 衡柳宏, 等. 鹅掌楸3个LcPIN1同源基因在体胚发生中的表达模式和功能分析[J]. 南京林业大学学报(自然科学版). 2025, 49(4): 57-70.
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
施季森. 迎接21世纪现代林木生物技术育种的挑战[J]. 中国农业科技导报, 2000, 2(1):36-41.
|
| [8] |
宗亦臣, 郑勇奇, 马锡权, 等. 湿加松良种‘中林1号’[J]. 湖南林业科技, 2017, 44(1):76-77.
|
| [9] |
胡继文, 郭文冰, 邓乐平, 等. 湿地松及其杂种的体细胞胚胎发生与植株再生[J]. 华南农业大学学报, 2019, 40(1):107-115.
|
| [10] |
|
| [11] |
|
| [12] |
胡珊, 杨春霞, 古振军, 等. 火炬松体细胞胚胎发生体系的优化[J]. 林业科学研究, 2022, 35(3):9-17.
|
| [13] |
|
| [14] |
张建伟, 王军辉, 李青粉, 等. 云杉未成熟合子胚诱导体细胞胚胎发生[J]. 林业科学, 2014, 50(4):39-46.
|
| [15] |
程方, 孙婷玉, 叶建仁. 抗松针褐斑病湿地松未成熟合子胚胚性愈伤组织的诱导[J]. 南京林业大学学报(自然科学版), 2023, 47(6):175-182.
|
| [16] |
|
| [17] |
|
| [18] |
刘建飞, 刘炎, 刘克俭, 等. 长白落叶松体胚发生再生体系优化[J]. 植物学报, 2020, 55(5):605-612.
|
| [19] |
|
| [20] |
宋跃, 甄成, 张含国, 等. 长白落叶松胚性愈伤组织诱导及体细胞胚胎发生[J]. 林业科学, 2016, 52(10):45-54.
|
| [21] |
任毓辉, 聂帅, 彭春雪, 等. 红松胚性愈伤组织增殖的激素配比、糖源类型和增殖周期效应研究[J]. 植物研究, 2022, 42(4):704-712.
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
/
| 〈 |
|
〉 |